Automation in Microfluidics: Real-Time Monitoring and Feedback Loops
As the field of microfluidics continues to evolve, automation is becoming essential for achieving precise, reproducible, and high-throughput results. Manual operation often falls short due to human variability and the need for constant adjustments. Automation in microfluidics combines advanced hardware and intelligent software to enable precise fluid handling with minimal user intervention. By introducing automated control, real-time flow monitoring, and adaptative feedback, these systems ensure consistency and high-performance microfluidic workflows.
From manual challenges to automated solutions in microfluidics
Traditional microfluidic experiments require constant adjustments to maintain stability in pressure or flow rate. Manual calibration introduces variability and slows down workflows, reducing experimental throughput and reproducibility. Even small variations can affect mixing efficiency, shear stress, or cell viability, which are critical in applications like organ-on-a-chip and long-term cell culture
Automation in microfluidics addresses these challenges by combining precise hardware with intelligent software control. Pressure controllers, valves, and flow sensors work together with real-time feedback algorithms to continuously monitor and adaptatively correct experimental parameters. When deviations occur, the system adjusts in real time, maintaining the target flow or pressure without any user intervention.
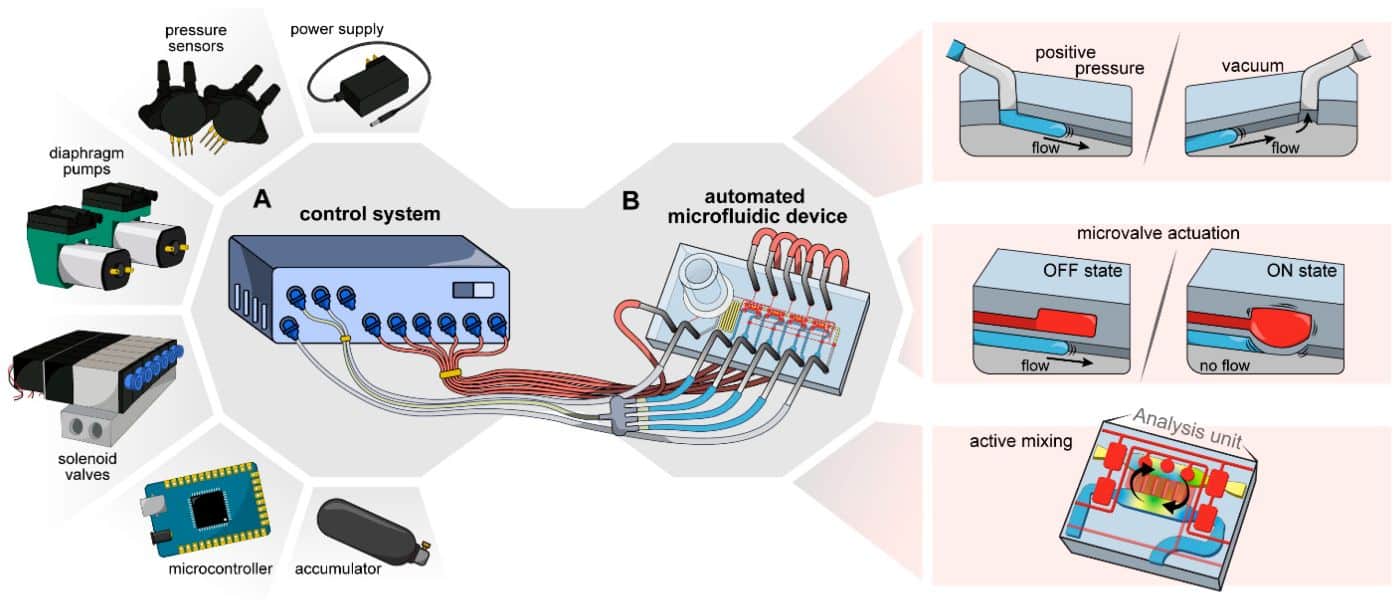
Figure 1. A control system for automated operation of microfluidic devices1
Fluigent is leading this transition toward automated microfluidic systems. Its suite of instruments, including the LineUp Series, MFCS Series, and Aria, enables automated and remote-controlled fluid handling. These platforms allow users to execute complex microfluidic protocols with minimal supervision, ensuring reproducibility and reducing human error.
To extend this flexibility even further, Fluigent provides a dedicated Software Development Kit (SDK) for advanced automation and system integration. The SDK enables the precise control of all Fluigent instruments through custom programs written in Python, LabVIEW, C++, C#, or MATLAB. The SDK includes practical examples ranging from basic operations, such as reading sensor data or setting pressure (fgt_get_sensorValue, fgt_set_pressure), to more advanced routines involving synchronized regulation and valve control. These examples make it easy to design customized automated workflows and integrate Fluigent devices into larger laboratory systems.
Real-time monitoring and feedback loops: enabling smart and adaptative microfluidics
At the core of automation in microfluidics lies the ability to monitor and adjust conditions in real time. By using integrated pressure and flow sensors, automated systems can detect deviations instantly and correct them through closed-loop feedback control, ensuring stable and reproducible experimental conditions.
Fluigent’s OxyGEN software plays a central role in this process. It continuously tracks pressure and flow rate from connected instruments and sensors, displaying them live while also adjusting system parameters. In an automated loop, OxyGEN can respond to any change, like clogging or fluid resistance, by automatically adjusting the pressure to maintain the target flow rate.
This process relies on Fluigent’s Direct Flow Control (DFC) algorithm. By combining flow sensors with responsive algorithms, DFC continuously compares measured flow rates to desired setpoints and dynamically adjusts the applied pressure to compensate for fluctuations. This microfluidic feedback loop ensures precise control even when environmental or sample conditions change, which is ideal for long-term perfusion or droplet-based applications.
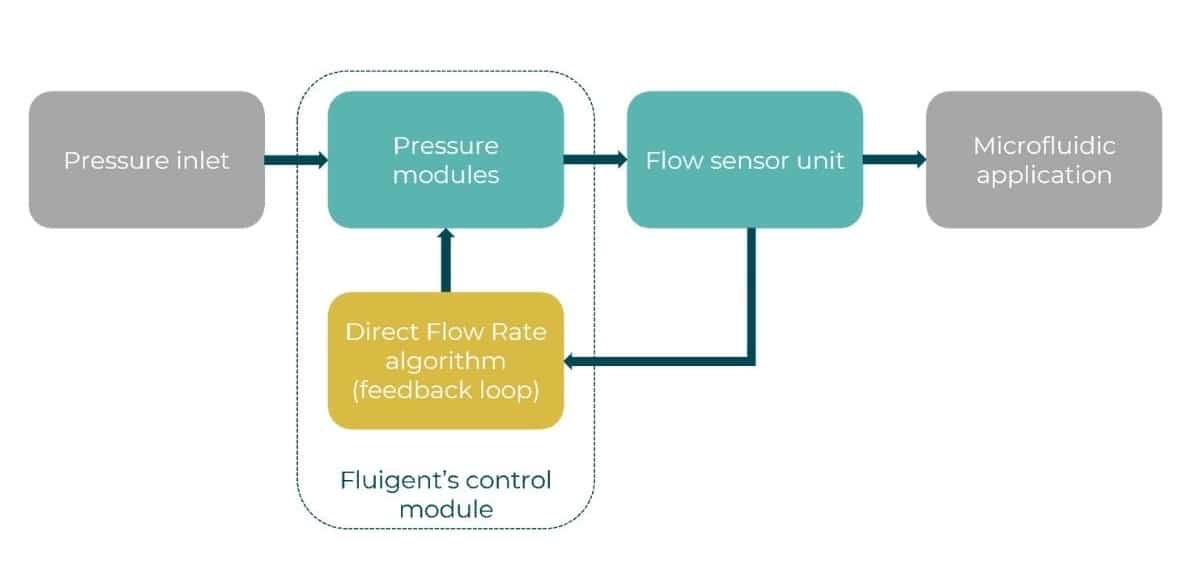
Figure 2. Operating principle of the microfluidic flow rate control algorithm. Both pressure and flow rates are monitored, and DFC automatically adjust pressures to maintain the target values.
Beyond simple monitoring, OxyGEN includes a Protocol Editor that automates complex microfluidic sequences. Users can create step-by-step routines directly within the software, define timing, loop operations, and conditional responses, and even simulate protocols before running them on actual hardware. This helps transform manual workflows into reproducible, automated protocols.
For example, in a recirculation experiment, OxyGEN controls valve positions to alternate flow direction and maintain constant fluid volume in the circuit during long-term cell culture. If flow resistance changes, DFC instantly compensates by adjusting pressure to maintain a stable rate. This fully automated feedback loop minimizes human intervention and ensures continuous, reliable perfusion over hours or days.
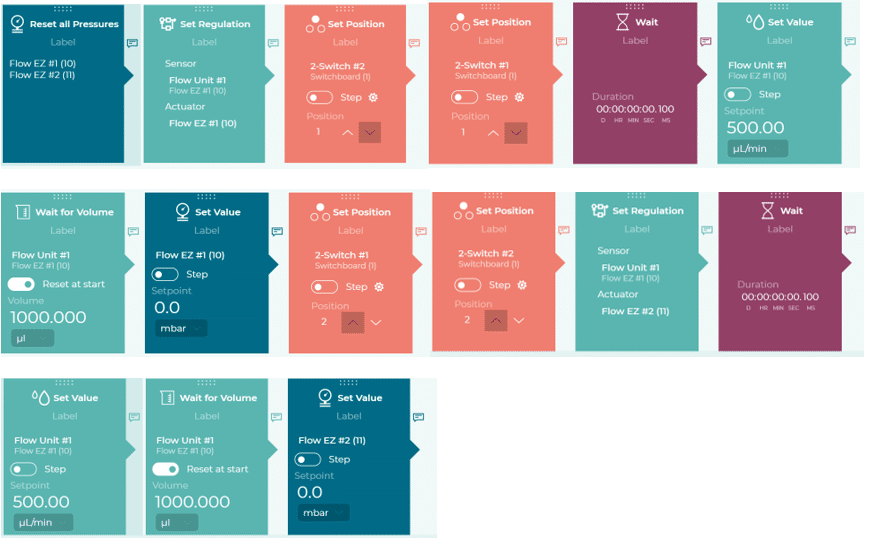
Figure 3. OxyGEN interface for recirculation protocol running
Figure 4. Fluid going back and forth between the two reservoirs during recirculation
Learn more about the Microfluidic Recirculation System
Applications and case studies of automation in microfluidics
The impact of automation in microfluidics extends across multiple domains, from biomedical research to chemical synthesis. Automated microfluidic systems are transforming workflows by improving control over delicate biological or chemical environments.
Organ-on-a-chip and cell culture
Automation is essential for maintaining stable physiological conditions in organ-on-a-chip and cell culture applications. Continuous perfusion replicates in vivo environments, ensuring cell viability and long-term stability.
Fluigent’s pressure-based flow controllers, combined with OxyGEN software, enable precise regulation of perfusion in real time over several weeks. Changes in flow resistance or fluid properties resulting from cellular growth are instantly compensated, maintaining optimal flow throughout the experiment.
For compact and fully integrated automation, the Omi platform combines pressure control, real-time monitoring, and adaptive feedback in a single incubator-compatible device. Omi supports long-term cell perfusion and recirculation with minimal user intervention, ensuring reproducibility across experiments.
These automated systems have been successfully implemented in multi-organ models, demonstrating precise flow control across connected chambers to mimic inter-organ interactions.
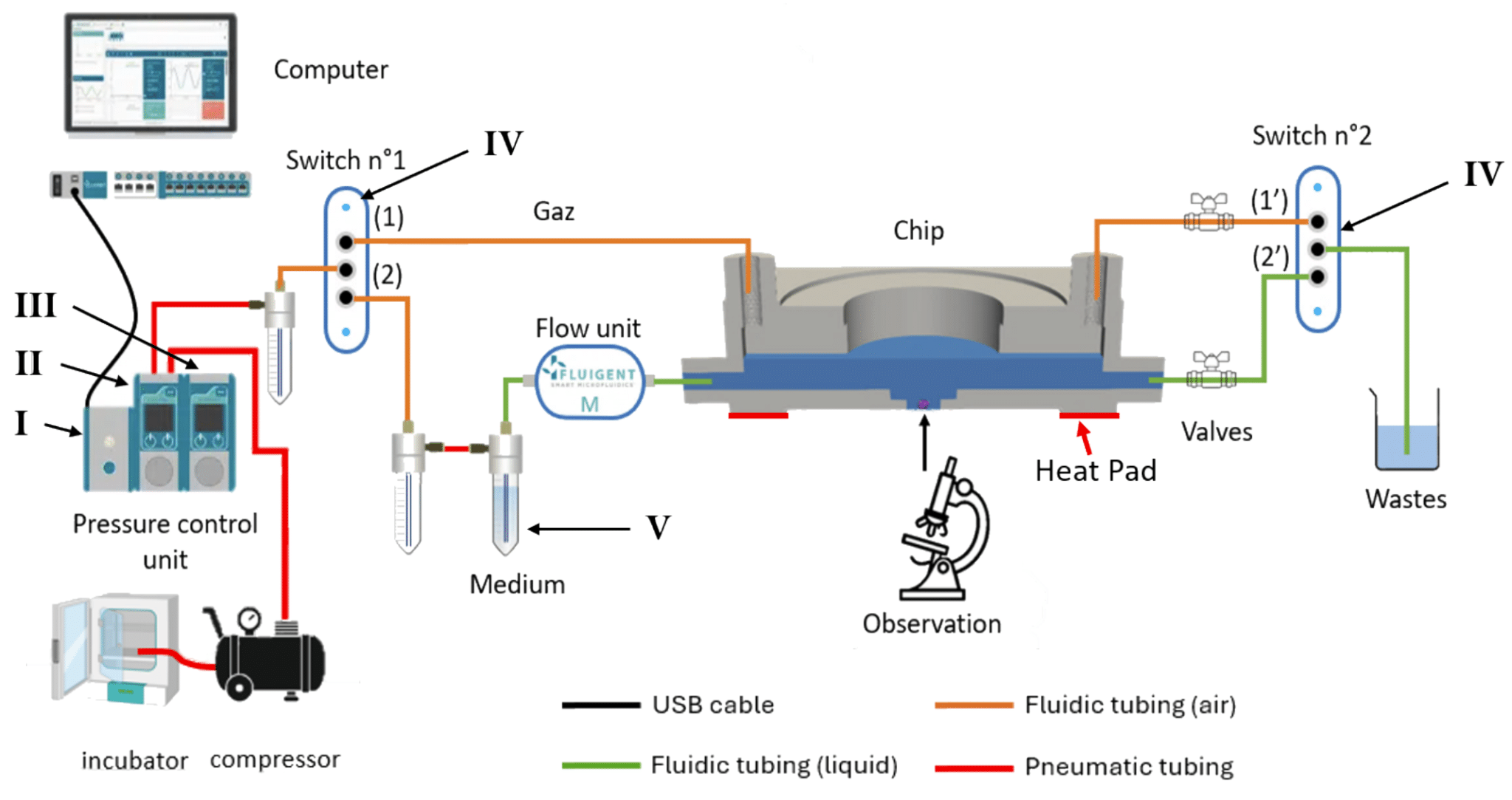
Figure 5. Automated platform for long-term cell culture in tumor research with Fluigent components: I-LineUp Link Module; II-LineUp PushPull (±1000/–800 mbar); III-LineUp Switch EZ; IV-two Switch modules; V-three 15 mL Pressure CAP HP reservoirs.2
Learn more on:
Liver-Kidney organ-on-a-chip model using the Omi dual platform
Microfluidic artery-on-a-chip using the MFCS
Drug delivery and automated screening
In pharmaceutical research, automated microfluidic workflows enable drug formulation, delivery, and screening in a reproducible way. Fluigent’s LineUp series, combined with OxyGEN software, allows for precise and automated preparation and delivery of drug formulations. For example, in the preparation of drug-loaded liposomes, automated control ensures consistent mixing ratios, flow rates, and encapsulation conditions, critical parameters for efficacy and size distribution.
This is especially valuable in high-throughput drug screening, where multiple compounds must be tested across varying concentrations and conditions. Automated systems can easily switch between solutions, maintain stable flow, and adapt to experimental feedback, ensuring reliable and repeatable results.
Learn more on:
Drug-loaded Liposome preparation using microfluidics
Automated immunofluorescence and staining protocols
Automated microfluidic systems have transformed how immunostaining and immunofluorescence assays are performed. Traditional staining requires manual pipetting, long incubation times, and precise timing, steps that can introduce human error.
Fluigent’s Aria and microfluidic valves automate injection and rinse sequences with high precision. This ensures reproducible reagent distribution and saves valuable time.
Automated immunostaining is particularly beneficial for delicate samples such as neuronal or tissue cultures, where consistency in timing and reagent concentration is critical to obtaining clear, reproducible fluorescence signals.
Learn more on:
Automated Immunofluorescence Application Note
Neuronal Cell Immunofluorescence Application Note
Droplet microfluidics
Droplet-based microfluidic workflows rely on precise synchronization between multiple fluid streams. Small flow variations can influence droplet size, frequency, or encapsulation efficiency.
Fluigent’s automated flow control solutions, using the DFC algorithm, ensure stable and consistent droplet formation, a key factor for single-cell analysis, drug screening, or material synthesis.
These systems simplify multi-channel droplet experiments, allowing researchers to run precise and long-term protocols with minimal supervision.
Learn more: Encapsulation and culture for single cell sequencing
Future perspectives: From automation to artificial intelligence
The integration of artificial intelligence (AI) represents the next evolution in microfluidic control. AI-driven systems could analyze real-time sensor data, predict deviations, and adjust parameters before instability occurs, achieving predictive control instead of reactive feedback.
Recent studies demonstrate the feasibility of integrating machine learning algorithms with automated microfluidic systems. Machine learning models can already predict droplet size and optimize chip geometries in silico.3
By combining data-driven algorithms, high-frequency sensors, and adaptive hardware, next-generation microfluidic systems will autonomously make experimental decisions, monitor performance, and even design optimized workflows, marking a shift toward intelligent, self-regulated microfluidic labs.
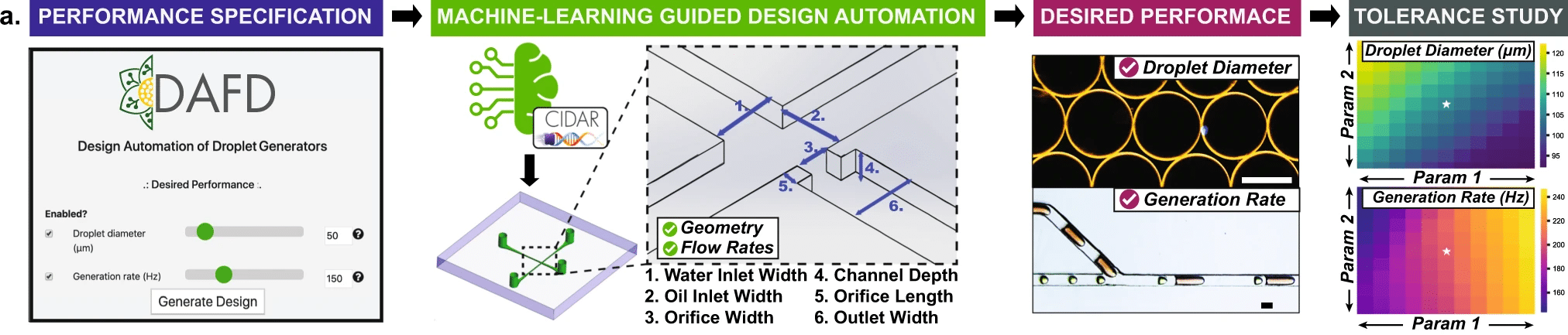
Figure 6. The workflow of a developed design automation tool for flow-focusing droplet generators, called DAFD.3
Conclusion
Automation in microfluidics is transforming how laboratories conduct experiments. By integrating precision hardware, smart algorithms, and real-time feedback, automated systems deliver consistency, scalability, and efficiency unmatched by manual operation.
Fluigent’s hardware and software ecosystem, including the MFCS, Flow EZ, OxyGEN, DFC algorithm, and Omi platform, exemplifies this transformation. Together, they create fully automated and reproducible microfluidic setups, enabling researchers to focus on science rather than system management.
Discover our range of flow control instruments
Expertises & Resources
-
Microfluidics Article Reviews An automated microfluidic platform for investigating mutation accumulation Read more
-
Product presentation videos MICROFLUIDIC VALVE AUTOMATION: How to make it easy [SWITCH EZ] – Fluigent Read more
-
Tutorial videos OxyGEN Automations Protocols: Container Blocks for Loops and Conditions Read more
-
Microfluidic Application Notes Development of a human gut-on-chip to assess the effect of shear stress on intestinal functions Read more
-
Microfluidic Application Notes Peristaltic Pump vs Pressure-Based Microfluidic Flow Control for Organ on Chip applications Read more
References:
(1) Gonzalez-Suarez, A. M.; Long, A.; Huang, X.; Revzin, A. A Compact Control System to Enable Automated Operation of Microfluidic Bioanalytical Assays. Biosensors 2022, 12 (12), 1160. https://doi.org/10.3390/bios12121160.
(2) Lacour, M.; Abdelwahed, A. B.; Azaiez, M.; Sciumè, G. Digitally Controlled Microfluidic System for 3D Cell Aggregate Cultures: Towards Advanced Modeling of Tumor Microenvironment. In 26e Congrès Français de Mécanique; 2025.
(3) Lashkaripour, A.; Rodriguez, C.; Mehdipour, N.; Mardian, R.; McIntyre, D.; Ortiz, L.; Campbell, J.; Densmore, D. Machine Learning Enables Design Automation of Microfluidic Flow-Focusing Droplet Generation. Nat. Commun. 2021, 12 (1), 25. https://doi.org/10.1038/s41467-020-20284-z.
(4) Liang, X.; Ouyang, M.; Brandon, N. P.; Xuan, J.; Wang, H. Automated Microfluidics for Efficient Characterization of Cyclohexanol Electrooxidation for Sustainable Chemical Production. JACS Au 2025, 5 (3), 1340–1349. https://doi.org/10.1021/jacsau.4c01207.
(5) Zhan, L.; Hinnen, H.; Gopinathan, K. A.; Toner, M. Autonomous Cryoprotectant Loading of the Oocyte Using Microfluidic Transistors. Device 2025, 3 (6).
(6) Chargueraud, A.; Kool, L.; Fattaccioli, J. Fully Integrated Automatic Reusable Microfluidic Setup for Immobilization, Analysis and Non-Selective Release of Particles. arXiv June 22, 2025. https://doi.org/10.48550/arXiv.2507.06241.
(7) Amador-Hernandez, J. U.; Gonzalez-Suarez, A. M.; Stybayeva, G.; Caballero-Robledo, G. A.; Garcia-Cordero, J. L.; Revzin, A. An Automated Thermoplastic Microfluidic Device for Rapid Analysis of Microliter Volumes of Blood. Available at SSRN 5192100.
(8) Wang, X. Compact and Automated Multi-Solution Controller for Microfluidic Devices. PhD Thesis, Johns Hopkins University, 2025. https://jscholarship.library.jhu.edu/items/dff6cf5a-4a58-4b9d-936c-bea73040e34c (accessed 2025-10-06).
(9) Osaid, M.; Marino Miguélez, M. H.; Baryak, B.; Özmen-Capin, B. B.; Özenci, V.; van der Wijngaart, W. Rapid Automated Isolation and Concentration of Bacteria from Blood Samples. bioRxiv 2025, 2025–03.