Peristaltic Pump vs Pressure-Based Microfluidic Flow Control for Organ on Chip applications
Using microfluidic technology for cell culture offers many benefits compared to traditional methods like flasks, Petri dishes, and well-plates.
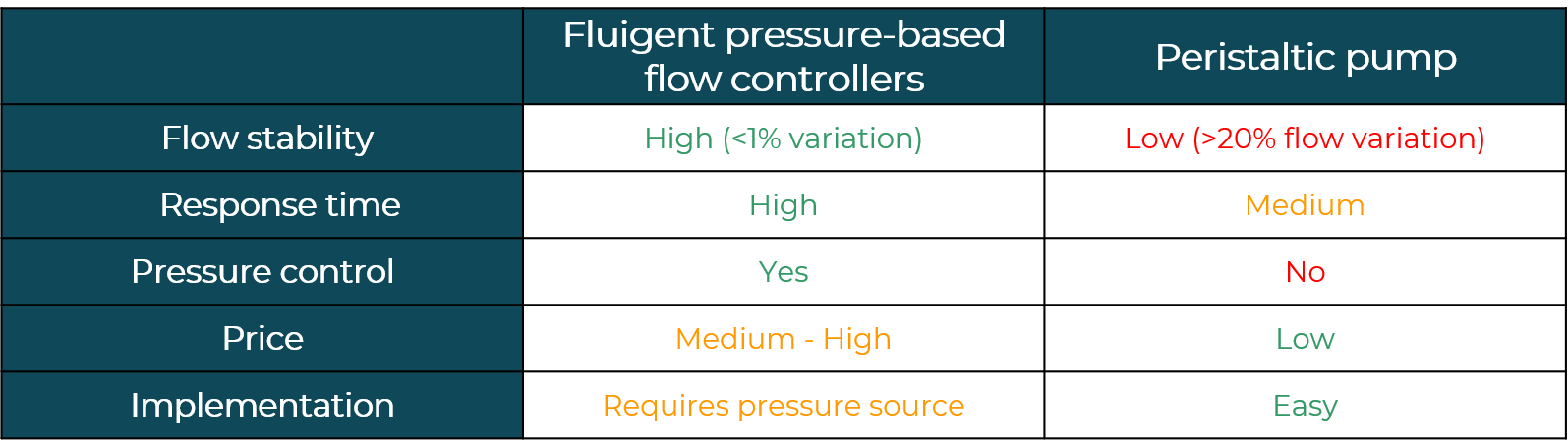
One crucial aspect of this technology is the type of perfusion system used. Peristaltic pumps, which are commonly employed, can cause damage to cells due to their unstable flow patterns that do not mimic real-life conditions. In contrast, pressure-based systems can produce steady flows or replicate realistic pulsatile flow patterns that simulate the aortic flow.
In this application note, the importance of flow stability in microfluidic systems is demonstrated by performing cell culture of endothelial cells in microfluidic chips and comparing results from using peristaltic pumps to results from pressure-based flow controllers.
Introduction
Pulsatile Peristaltic Pump for Organ on Chip: Low stability & response time
Flow control for Organ on Chip is a critical aspect of creating functional microphysiological systems. Historically, peristaltic pumps have been widely utilized to manage liquid flows due to their versatility and ease of use. However, in recent years, researchers have begun to shift towards the utilization of pressure-driven flow control systems. This shift in preference is due to the fact that peristaltic pumps cannot be modelled as perfect flow rate generators, as the back pressure generated by the pump results in a decrease in flow rate.
An alternative to peristaltic pumps, known as “piezo-electric pumps,” can be employed for intermediate flow rate applications (µL). However, for flow control of Organ on Chip applications, these pumps require the integration of a flow rate sensor, and fluctuations in flow rate can be observed at lower flow rates. High-performance liquid chromatography (HPLC) pumps, although effective in minimizing these fluctuations, can be quite costly.
Pressure-based flow controllers for Organ on Chip applications
A more recent development in liquid flow control is the utilization of microfluidic pressure controllers, which can pressurize a tank containing the sample, and smoothly and almost instantaneously inject it into a microfluidic chip. By coupling a pressure controller with a flow sensor, ultra-precise and sensitive flow control can be achieved. Additionally, by utilizing specialized software such as OxyGEN, researchers can monitor the entire process to ensure that the desired results are obtained.
Peristaltic Pump vs Pressure-Based flow controllers
Flow control for Organ on Chip is crucial to maintaining liquid flow stability in long-term experiments. Although peristaltic pumps offer the ability to create a closed loop of liquid flow, they have some limitations in terms of stability over time, making it necessary to repeatedly calibrate the flow rate. Additionally, the pulse issue at low flow rates is significantly higher than with syringe pumps.
In contrast, modern microfluidic pressure controllers offer a higher degree of precision and stability in flow rate control, and are particularly useful when working with dead-end channels or large sample volumes. As such, they have become increasingly popular for experiments where flow control for Organ on Chip applications calls for high flow responsiveness, stability and accuracy.
To demonstrate the importance of flow stability in vascular models, endothelial cells were seeded in microfluidic chips and then perfused either using a peristaltic pump or pressure-based flow controllers.
Materials and methods
Fluid recirculation system
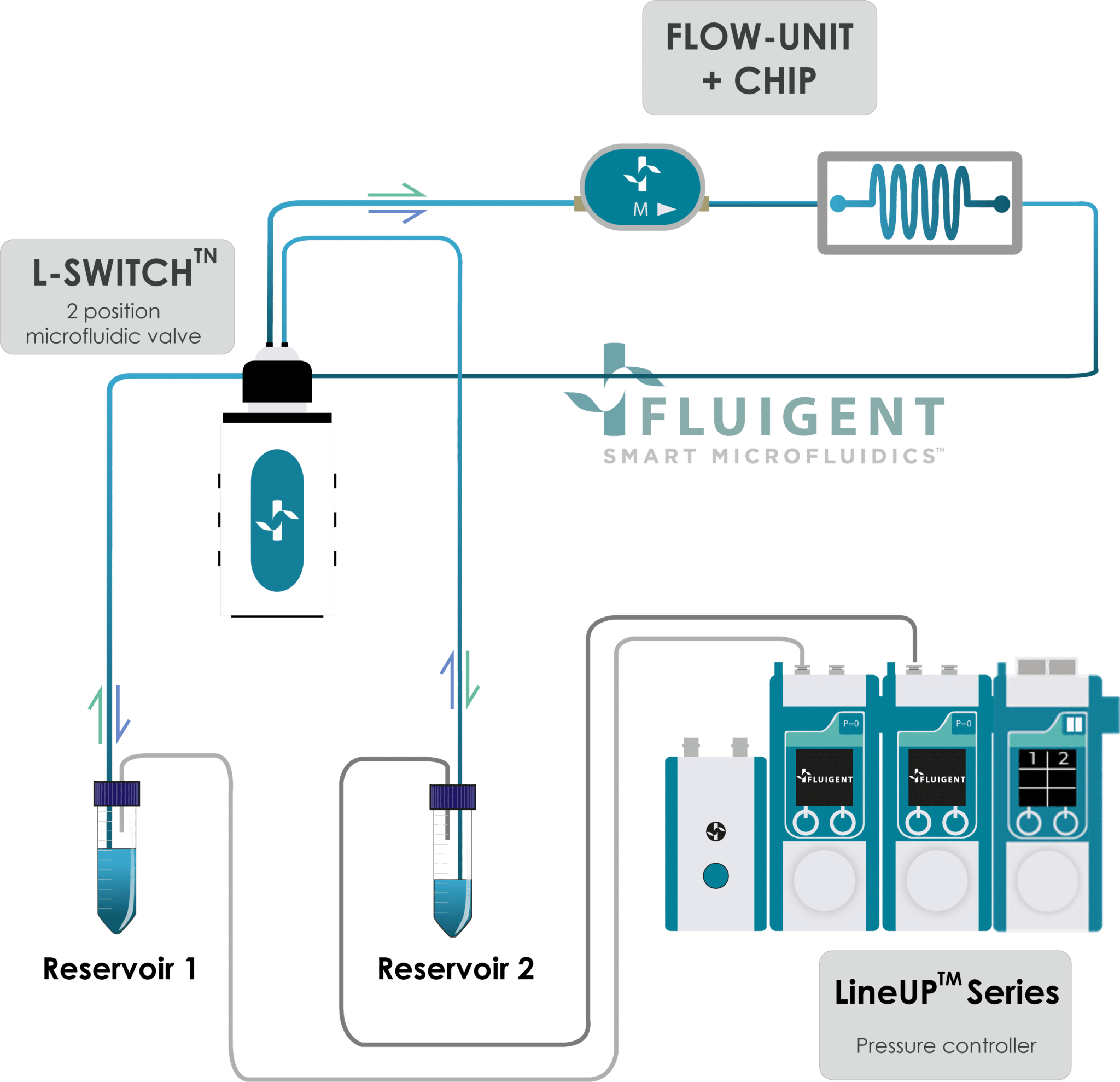
Figure 2 shows the operating principle of the recirculation system built with pressure-based flow controllers for our study of the importance of flow control in organ on chip applications. Two Flow EZ devices are connected to two reservoirs. Tubing passes through the L-SWITCH (allowing media recirculation), a flow unit, and the microfluidic device.
In the system using the peristaltic pump, the inlet and outlet tubing are both placed in a reservoir containing media which continuously flows within the microfluidic device. In both systems, the flow rate was monitored with a Flow Unit to evaluate fluctuations.
Results
HBEC-i cells are seeded at ~80% confluency in both chips. Before flowing liquid to the microfluidic devices, the two cell cultures are similar in term of viability and confluency.
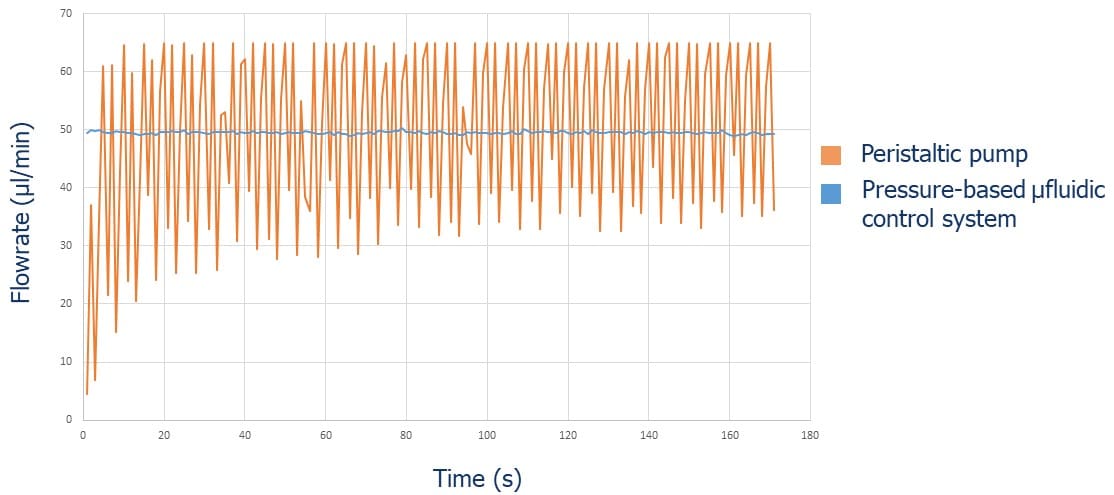
In this experiment, which addresses the importance of flow control in organ on chip applications, culture medium is recirculated to ensure a continuous supply of nutrients and O2 to the cells. Medium recirculation is performed using the Flow EZ (pressure-based flow controllers) or a peristaltic pump in each microfluidic device with a set flow rate of 50 µl/min for t = 24h. Figure 2 shows the flow rate over time using the peristaltic pump (in orange) and the Flow EZ (in blue). Using the peristaltic pump, the flow rate fluctuates greatly, more than 40% variation in flow rate compared to the target value.
When using the Flow EZ, we observe a highly stable flow rate with less than 2% flow variation.
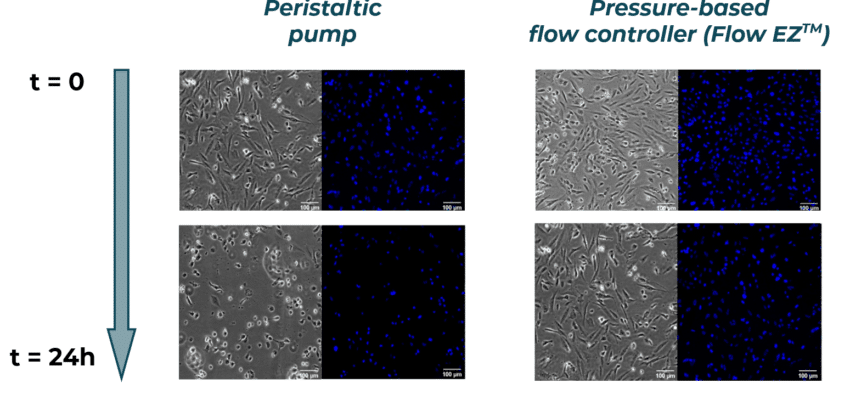
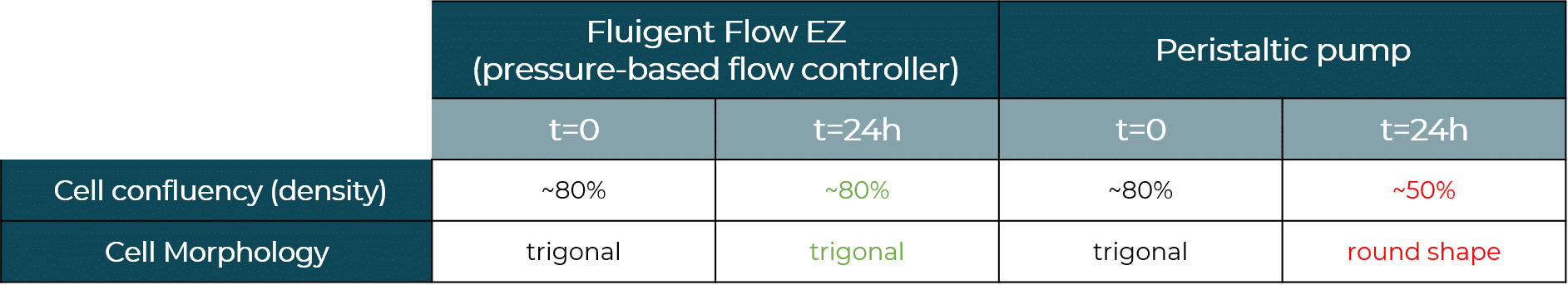
After 24h of media recirculation, we observe a decrease in cell density with the peristaltic pump when compared to t=0, suggesting that the large flow rate fluctuations have led to cell detachment. Cells exhibit a round shape, as opposed to the well spread cells with trigonal shape observed before perfusion. This suggests that, even when not detached, cells show less adhesion and are less viable, and cell function might have been impacted by the poor flow conditions.
In the cell culture perfused with the Flow EZ, we observe a similar cell confluency of ~80% when compared to t=0. In addition, cells have similar spreading, with trigonal shape. These results confirm that cells perfused with the Flow EZ remained healthy and viable due to ideal flow conditions.
Conclusion
When transitioning from traditional flask-based cell culture to microfluidics, much of our attention is directed toward the microfluidic chip. However, we also need to focus on reproducing and controlling flows that are suitable for organ-on-chip applications and allow for replication of in vivo conditions, which is crucial for successful outcomes. The choice of equipment used to emulate the flow conditions experienced by cells in vivo is crucial, since it can impact cell survival, phenotype and genetic expression.
The findings described in this application note were remarkable. Vascular cells that were cultured under irregular pulsatile flow died after only one day of perfusion. However, when subjected to a consistent laminar shear stress, comparable to real-life conditions, the cells survived and spread nicely in the microfluidic chip.
Related resources
- Expert Reviews: Basics of Microfluidics
Flow Control Technologies: Comparison between peristaltic, syringe and pressure pumps for microfluidic applications
Read more 
5 reasons to choose OEM pressure controllers over OEM syringe pumps for microfluidic applications
Read more
Microfluidics for Organ-on-chip Cell culture
Read more- Expert Reviews: Basics of Microfluidics
Flow control for droplet generation using syringe pumps and pressure-based flow controllers
Read more
Related products
-
 Microfluidic Leakage Testing Pack Read
Microfluidic Leakage Testing Pack Read -
 Microfluidic Flow Management Unit Read
Microfluidic Flow Management Unit Read -
 Omi, an Automated Organ-On-A-Chip Platform Read
Omi, an Automated Organ-On-A-Chip Platform Read -
 Modular OEM Microfluidic Flow Controller Read
Modular OEM Microfluidic Flow Controller Read -
 Microfluidic Injection Valve Read
Microfluidic Injection Valve Read -
 Microfluidic flow controller Read
Microfluidic flow controller Read -
 Microfluidic Recirculation Valve Read
Microfluidic Recirculation Valve Read -
 Microfluidic cell culture chip Read
Microfluidic cell culture chip Read -
 Double channel Microfluidic chip for hypoxic cell culture Read
Double channel Microfluidic chip for hypoxic cell culture Read -
 Organ on Chip Perfusion Pack Read
Organ on Chip Perfusion Pack Read -
 High Throughput Cell Perfusion Pack Read
High Throughput Cell Perfusion Pack Read -
 Microfluidic OEM Pressure Controller Read
Microfluidic OEM Pressure Controller Read -
 Fully Custom Microfluidic Device Read
Fully Custom Microfluidic Device Read -
 Sample injection and recirculation microfluidic valve for industry Read
Sample injection and recirculation microfluidic valve for industry Read