Microgel Crosslinking: Materials, Methods, and Emerging Applications
This review explores microgel crosslinking methods using natural polymers including alginate, agarose, gelatin, and more. Crosslinking techniques, including ionic, temperature-induced, chemical, and UV are discussed for tailoring microgel properties. Crosslinked microgels show great potential in tissue engineering, drug delivery, and cell encapsulation, offering precise control of mechanical properties, degradation rate, and molecular release for advanced biomedical applications.
Introduction to Microgel Crosslinking
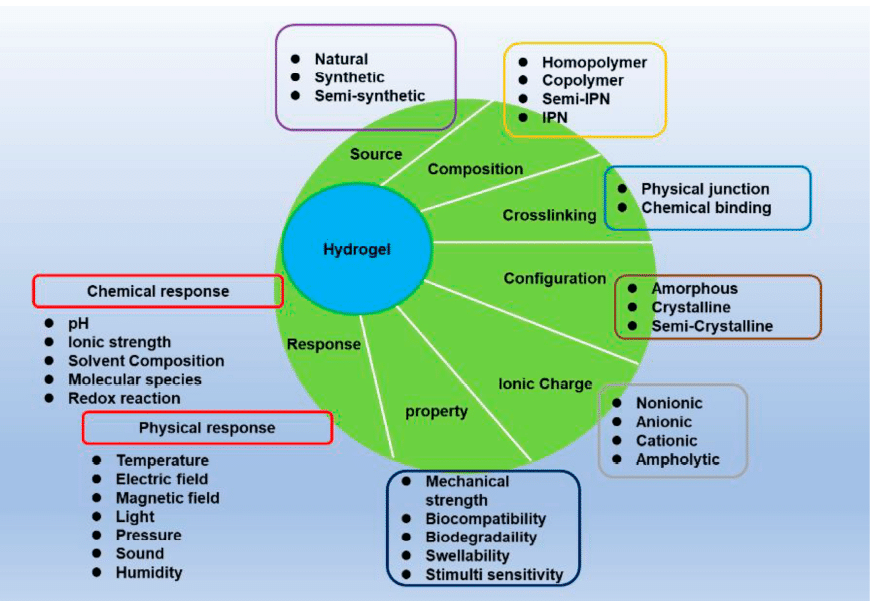
Hydrogels are three-dimensional networks of hydrophilic polymers that absorb and retain water while remaining insoluble once crosslinked. Their high-water content and soft, porous structure closely resemble the extracellular matrix (ECM), making them ideal for a wide range of applications, from drug delivery systems and tissue engineering scaffolds to wound dressings, hygiene products, and food packaging.1–4 Hydrogels can undergo reversible volume changes in response to environmental stimuli such as pH, temperature, or ionic strength, allowing for the manipulation of their physical properties (figure 1).
When scaled down , hydrogels form microgels; micron-sized particles that retain the functional properties of larger hydrogels but with enhanced control over their size, structure, and encapsulation. Microgels can be synthesized using methods like emulsification, micromolding, and microfluidics. Among these, microfluidic platforms offer superior control over particle size and morphology, enabling the production of uniform, monodisperse microgels with high encapsulation efficiency.1,5,6
This review will focus on microgel crosslinking methods, exploring how different polymer and crosslinking strategies are employed to create microgels with controlled properties.
Types of Microgels Based on Natural Polymers
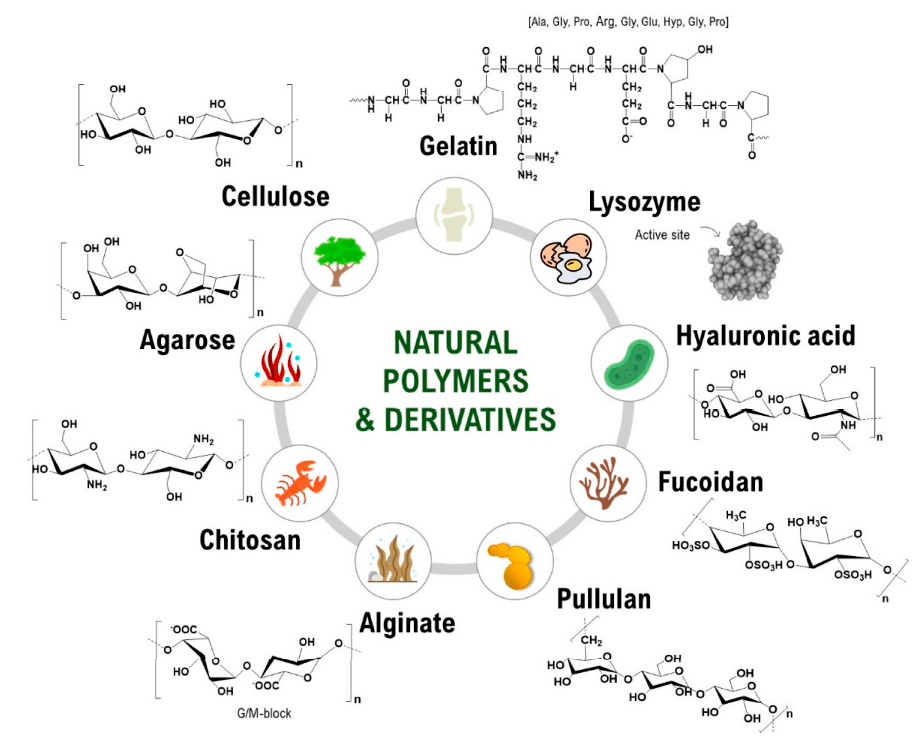
Natural polymers are commonly utilized in microgel synthesis due to their intrinsic biocompatibility and chemical versatility, which allow fine-tuning of their physicochemical properties. These polymers can be broadly categorized into polysaccharides and proteins, each providing distinct molecular architectures and reactive sites that are advantageous for microgel crosslinking and customization (figure 2).6
Polysaccharide-Based Microgels
Polysaccharides represent one of the most extensively explored classes of natural polymers for microgel fabrication. Alginate, a linear copolymer composed of mannuronic (M) and guluronic (G) acid residues, exhibits gelation like behavior that depends strongly on the ratio and distribution of these blocks. This structural variability directly impacts mechanical properties such as swelling behavior, and degradation rates of the resulting microgels. The mild gelation conditions and abundance of modifiable carboxyl groups make alginate an excellent candidate for generating tunable microgel networks.7
Chitosan, another widely studied polysaccharide, is derived from the partial deacetylation of chitin and possesses a cationic backbone that facilitates electrostatic interactions with negatively charged biomolecules or polyanions. Its functional amino groups enable chemical conjugation, blending with other polymers, and modulation of degradation rates. These features allow chitosan-based microgels to be engineered with controlled structural properties and enhanced bioactive potential.8,9
Protein-Based Microgels
Proteins offer a complementary set of properties for microgel fabrication, notably their bioactive sequences and hierarchical assembly patterns. Collagen, the most abundant structural protein in connective tissues, provides a triple-helical configuration that contributes to the mechanical stability and organization of protein-based microgels. This structure also contains cell-recognition, receptors, making collagen microgels structurally compatible with cellular environments.10
Gelatin, a denatured derivative of collagen, retains many of the bioactive sequences of its precursor but is more easily processed due to its solubility and lower antigenicity. Despite its relatively low mechanical strength, gelatin can be chemically modified or blended with other polymers to enhance stability. The widely used derivative gelatin methacryloyl, introduces methacryloyl functional groups capable of participating in photopolymerization reactions. This modification provides precise control over crosslinking density and enables the fabrication of microgels with tunable stiffness, degradability, and molecular architecture.6,11
Crosslinking Microgels from Natural Polymers
Crosslinking transforms natural polymer solutions into three-dimensional hydrogels by forming physical or covalent bonds between polymer chains. This process increases structural stability and tunes degradation behavior, which are crucial for biomedical applications. The appropriate method depends on the polymer chemistry, desired mechanical strength, and compatibility with biological components.1,5,6
Ionic Crosslinking
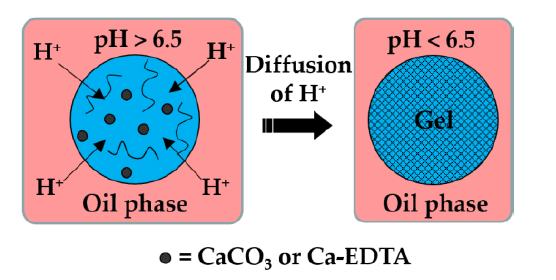
Ionic crosslinking is widely applied to polymers with charged functional groups, such as alginate (figures 3-4). In this method, droplets are formed within a continuous oil phase that contains an organic acid (often acetic acid) combined with hydrophobic surfactants. Inert carrier oils (example: vegetable oils, dimethyl carbonate, fluorocarbon oil) are typically used to stabilize this phase. Once the droplets are generated, the acid gradually migrates across the oil–water interface, where it dissociates into protons and acetate ions. The released protons react with calcium or barium salts present in the droplets, liberating Ca²⁺ or Ba²⁺ ions that subsequently interact with the polymer’s carboxylate groups, producing a crosslinked microgel network under mild conditions.
While this approach is rapid and biocompatible, the resulting gels may undergo gradual weakening in physiological fluids due to ion exchange with surrounding monovalent salts.1,7,12,13
Discover our Alginate Production Station for precise microbead fabrication
Temperature-Induced Gelation
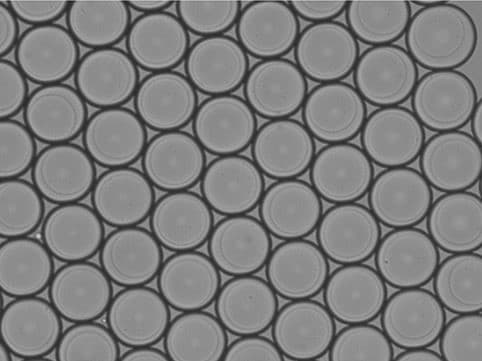
Certain polymers, including agarose and gelatin, undergo sol–gel transitions when the temperature changes. Agarose remains in liquid form at elevated temperatures (around 90 °C) and gels upon cooling (approximately 35–40 °C) as polymer chains associate via hydrogen bonding and hydrophobic interactions (figure 5-6). Gelatin, derived from collagen, similarly gels upon cooling through partial helix formation, though its gels are thermoreversible and may soften at body temperature unless stabilized further. This method is simple, additive-free, and highly biocompatible, but the mechanical strength of these gels is generally lower than covalently crosslinked systems.11,14–16
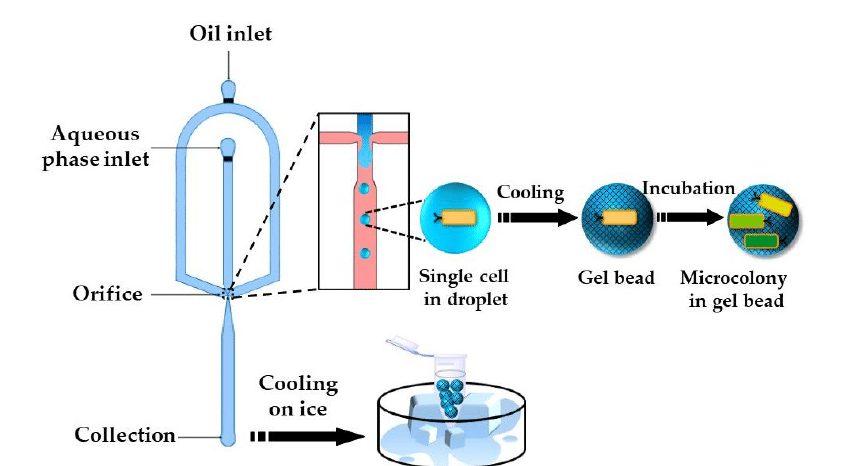
Photo-Crosslinking
Photo-crosslinking creates covalent bonds through light-initiated reactions. For example, poly(ethylene glycol) diacrylate (PEGDA) forms hydrogels when exposed to UV or visible light in the presence of a photoinitiator (figure 7). Light activation generates free radicals that polymerize the acrylate groups, rapidly solidifying the material. This method provides precise control over the location and timing of gelation and supports patterned or layered hydrogel fabrication. However, careful control of light intensity and exposure is required to avoid damaging sensitive biological molecules.1,17
Discover our all-in-one platform for continuous generation of UV-cured core-shell microcapsules.
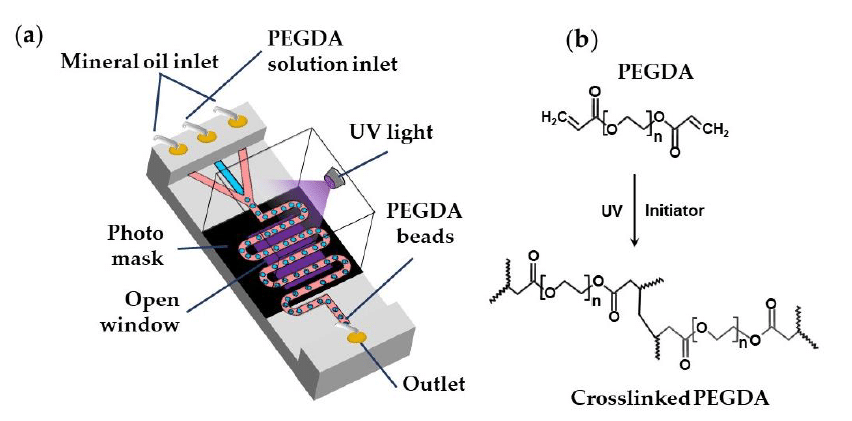
Chemical Crosslinking
Chemical crosslinking involves forming covalent bonds using bifunctional reagents. Proteins such as gelatin or collagen can be crosslinked with agents similar to glutaraldehyde, which react with available amino groups to create stable networks. This significantly improves mechanical properties and slows enzymatic degradation. Unreacted crosslinkers can be cytotoxic, so extensive washing or alternative low-toxicity crosslinkers may be required for biomedical applications.18
Enzymatic Crosslinking
Enzymatic methods utilize natural catalysts, such as transglutaminase or horseradish peroxidase (HRP), to form covalent bonds under mild conditions. Alginate can be modified with tyramine via carbodiimide chemistry and subsequently crosslinked using HRP and hydrogen peroxide, forming C–C and C–O bonds between phenol groups. This process can also be applied in droplet-based systems, where alginate-tyramine, cells, and HRP are encapsulated within droplets in a co-flowing stream of hydrogen peroxide–saturated paraffin (figure 8).1,19
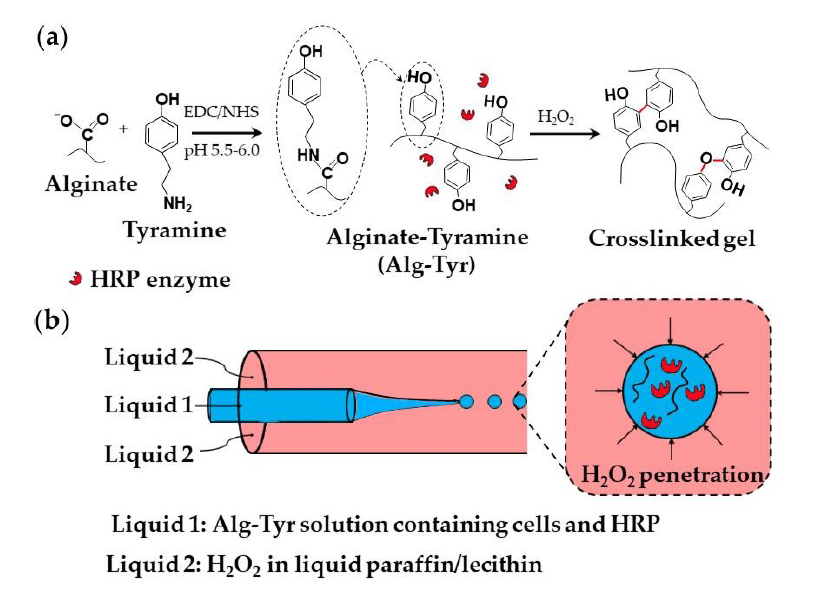
Applications for Microgels
Microgels offer advantages in tissue engineering by providing ECM-like scaffolds that support cell growth and tissue regeneration. Their customizable properties make them ideal for controlled cell encapsulation and drug delivery. This enables the sustained release of therapeutic agents with high precision. Additionally, microgels are being explored in bioactive molecule delivery, where they protect sensitive compounds and respond to environmental triggers. Their versatility extends to 3D bioprinting, where they contribute to the creation of intricate, stable structures for tissue reconstruction.1,5,6
| Characteristics | Description | Application in Microgels |
|---|---|---|
| Biocompatibility | Interaction with biological tissues without immune response. | Enables safe encapsulation of cells or drugs without causing immune rejection.7,20,21 |
| Biodegradability | Degradation into non-toxic byproducts. | Allows microgels to degrade naturally, eliminating the need for surgical removal.22,23 |
| Controlled Release | Sustained release of drugs or bioactive molecules. | Provides precise and sustained drug delivery over extended periods.24–26 |
| Stimuli-Responsiveness | Response to environmental stimuli (pH, temperature). | Enables targeted drug release in specific environments like tumors.27–29 |
| Mechanical Properties | Structural integrity under external forces. | Ensures microgels maintain shape and functionality in dynamic physiological conditions.30–32 |
Conclusion
Microgel crosslinking plays an important role in advancing applications in tissue engineering, encapsulation, and drug delivery by offering precise control over the structure and properties of microgels. The integration of microfluidic fabrication methods enables the production of highly uniform, functional microgels that closely replicate the extracellular matrix and enhance cell behavior.
Despite challenges in scalability and mechanical strength, continued advancements in microgel crosslinking methods offer promising potential for future clinical and therapeutic applications.
👉 Ready to advance your microgel research? Contact our experts or explore our microfluidic systems.
Related Products
Related Resources
-
Expert Reviews: Basics of Microfluidics Microfluidics in Drug Delivery: A New Era of Precision Medicine Read more
-
Expert Reviews: Basics of Microfluidics 10 Tips for Reliable Droplet Generation Read more
-
Microfluidics White Papers Double emulsion for the generation of microcapsules – a Review Read more
-
Microfluidic Application Notes UV-Crosslinking of Microcapsules Read more
-
Expert Reviews: Basics of Microfluidics Flow control for droplet generation using syringe pumps and pressure-based flow controllers Read more
References
1. Chen, M., Bolognesi, G. & Vladisavljević, G. T. Crosslinking Strategies for the Microfluidic Production of Microgels. Molecules 26, 3752 (2021).
2. Kamoun, E. A., Kenawy, E.-R. S. & Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 8, 217–233 (2017).
3. Kabir, S. M. F. et al. Cellulose-based hydrogel materials: chemistry, properties and their prospective applications. Prog. Biomater. 7, 153–174 (2018).
4. Batista, R. A. et al. Hydrogel as an alternative structure for food packaging systems. Carbohydr. Polym. 205, 106–116 (2019).
5. Chelu, M., Calderon Moreno, J. M., Musuc, A. M. & Popa, M. Natural Regenerative Hydrogels for Wound Healing. Gels 10, 547 (2024).
6. Rosellini, E. & Cascone, M. G. Microfluidic Fabrication of Natural Polymer-Based Scaffolds for Tissue Engineering Applications: A Review. Biomimetics 8, 74 (2023).
7. Lee, K. Y. & Mooney, D. J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 37, 106–126 (2012).
8. Ahsan, S. M. et al. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 110, 97–109 (2018).
9. Chachanidze, R., Xie, K., Lyu, J., Jaeger, M. & Leonetti, M. Breakups of Chitosan microcapsules in extensional flow. J. Colloid Interface Sci. 629, 445–454 (2023).
10. Parenteau-Bareil, R., Gauvin, R. & Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 3, 1863–1887 (2010).
11. Naharros‐Molinero, A., Caballo‐González, M. Á., De La Mata, F. J. & García‐Gallego, S. Shell Formulation in Soft Gelatin Capsules: Design and Characterization. Adv. Healthc. Mater. 13, 2302250 (2024).
12. Capretto, L., Mazzitelli, S., Balestra, C., Tosi, A. & Nastruzzi, C. Effect of the gelation process on the production of alginate microbeads by microfluidic chip technology. Lab. Chip 8, 617 (2008).
13. Enck, K. et al. Design of an Adhesive Film-Based Microfluidic Device for Alginate Hydrogel-Based Cell Encapsulation. Ann. Biomed. Eng. 48, 1103–1111 (2020).
14. Eun, Y.-J., Utada, A. S., Copeland, M. F., Takeuchi, S. & Weibel, D. B. Encapsulating Bacteria in Agarose Microparticles Using Microfluidics for High-Throughput Cell Analysis and Isolation. ACS Chem. Biol. 6, 260–266 (2011).
15. Leng, X., Zhang, W., Wang, C., Cui, L. & Yang, C. J. Agarose droplet microfluidics for highly parallel and efficient single molecule emulsion PCR. Lab. Chip 10, 2841–2843 (2010).
16. Digenis, G. A., Gold, T. B. & Shah, V. P. Cross‐Linking of Gelatin Capsules and Its Relevance to Their in Vitro-in Vivo Performance. J. Pharm. Sci. 83, 915–921 (1994).
17. Hakim Khalili, M. et al. Additive Manufacturing and Physicomechanical Characteristics of PEGDA Hydrogels: Recent Advances and Perspective for Tissue Engineering. Polymers 15, 2341 (2023).
18. Yamada, M. et al. Cell-sized condensed collagen microparticles for preparing microengineered composite spheroids of primary hepatocytes. Lab. Chip 15, 3941–3951 (2015).
19. Sakai, S., Hashimoto, I., Ogushi, Y. & Kawakami, K. Peroxidase-Catalyzed Cell Encapsulation in Subsieve-Size Capsules of Alginate with Phenol Moieties in Water-Immiscible Fluid Dissolving H2O2. Biomacromolecules 8, 2622–2626 (2007).
20. Ashimova, A., Yegorov, S., Negmetzhanov, B. & Hortelano, G. Cell Encapsulation Within Alginate Microcapsules: Immunological Challenges and Outlook. Front. Bioeng. Biotechnol. 7, (2019).
21. Håti, A. G. et al. Versatile, cell and chip friendly method to gel alginate in microfluidic devices. Lab. Chip 16, 3718–3727 (2016).
22. Cheng, H. et al. Long-term antibacterial and biofilm dispersion activity of an injectable in situ crosslinked co-delivery hydrogel/microgel for treatment of implant infection. Chem. Eng. J. 433, 134451 (2022).
23. L Palmese, L. et al. Injectable liposome-containing click hydrogel microparticles for release of macromolecular cargos. Soft Matter (2024) doi:10.1039/D3SM01009K.
24. Mora-Boza, A., Ahmedin, Z. & García, A. J. Controlled release of therapeutic antibody using hydrolytically degradable microgels. J. Biomed. Mater. Res. A 112, 1265–1275 (2024).
25. Singh, N. et al. Chitosan Hydrogels with Embedded Thermo- and pH-Responsive Microgels as a Potential Carrier for Controlled Release of Drugs. ACS Appl. Bio Mater. 5, 3487–3499 (2022).
26. Jin, H., Wen, J., Wang, L., Zhang, Y. & Sui, X. Synthesis and characterization of ion-induced sodium alginate/soy protein isolate microgels for the controlled release. Food Chem. 452, 139588 (2024).
27. Kulkarni, A. S., Tapase, S. R., Kodam, K. M. & Shinde, V. S. Thermoresponsive Pluronic based microgels for controlled release of curcumin against breast cancer cell line. Colloids Surf. B Biointerfaces 205, 111834 (2021).
28. Agnihotri, P., Sangeeta, Aery, S. & Dan, A. Temperature- and pH-responsive poly(N-isopropylacrylamide-co-methacrylic acid) microgels as a carrier for controlled protein adsorption and release. Soft Matter 17, 9595–9606 (2021).
29. Jeong, H.-S. et al. Broad-temperature-range mechanically tunable hydrogel microcapsules for controlled active release. J. Controlled Release 356, 337–346 (2023).
30. Schulte, M. F. et al. Microgels react to force: mechanical properties, syntheses, and force-activated functions. Chem. Soc. Rev. 51, 2939–2956 (2022).
31. Coronel, M. M. et al. Hydrolytically Degradable Microgels with Tunable Mechanical Properties Modulate the Host Immune Response. Small 18, 2106896 (2022).
32. Wang, X. et al. 3D bioprinting microgels to construct implantable vascular tissue. Cell Prolif. 56, e13456 (2023).