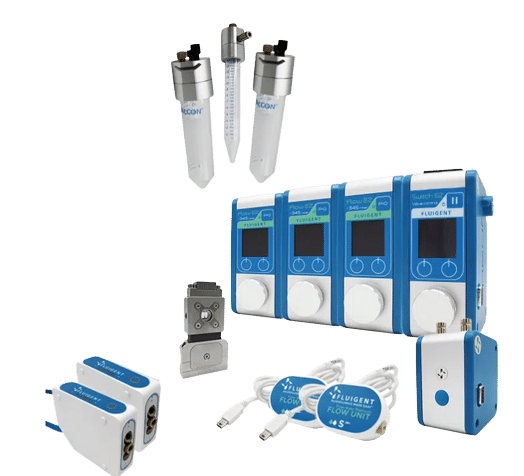
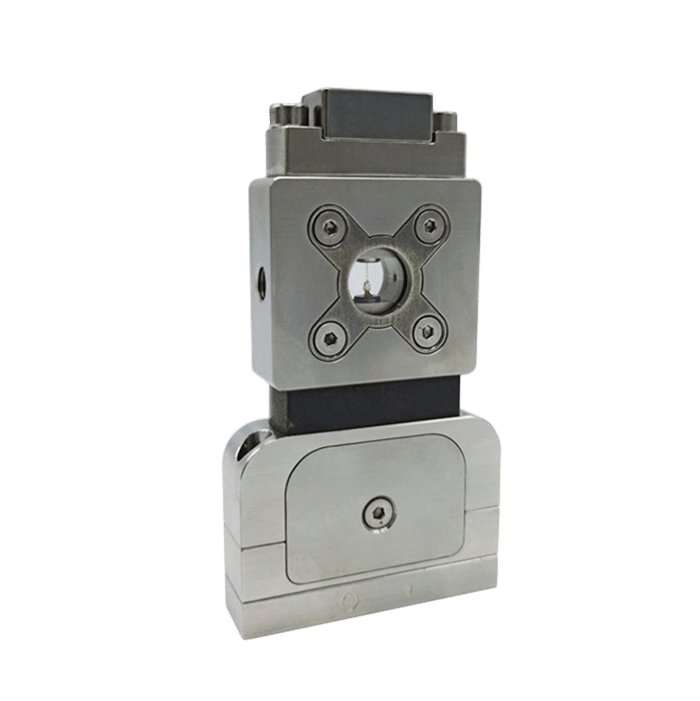
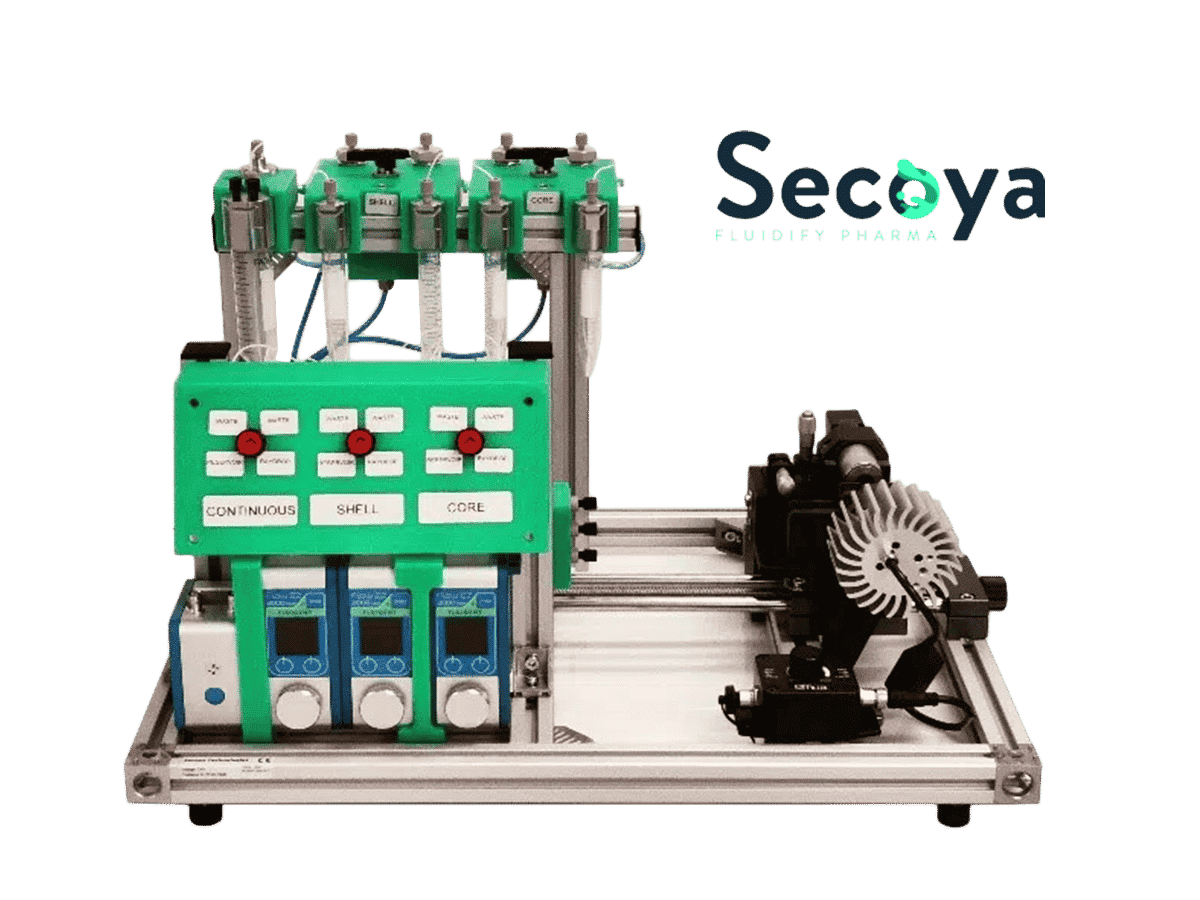
UV-Crosslinking of Microcapsules
Core-shell microcapsules combine the features of both the core and shell materials, while exhibiting smart properties resulting from their materials. The novel properties make them extremely suitable for pharmaceutical and biomedical applications, including cell encapsulation, cell study, targeted drug delivery, controlled drug release, food industry, catalysis, and environmental monitoring.
Using Fluigent’s pumping technology and the RayDrop Double Emulsion, developed and manufactured by Secoya, controlled double emulsification is achieved by dripping or jetting the core fluid into an immiscible shell fluid, which is then encapsulated by the third fluid.
In this application note, we describe the UV-crosslinking of microcapsules process, in which microcapsules are formed by consolidating shell phase of the resulting double emulsions by uv-crosslinking of monomers and photoinitiator used as shell phase.
Secoya developed and manufactured the RayDrop used to perform this application note.

Why use microcapsule ?
Over the past few decades, core-shell microcapsules have played an important role in the delivery and release of materials in the pharmaceutical, cosmetic, and food industries. Such applications often require a high degree of control over chemical release, a quality directly related the capsule material as well as the homogeneity of the capsule size and shell thickness.
Methods of microcapsules production
Microcapsules can be produced using a variety of methods, including polymerization, spray drying, solvent evaporation, and self-assembly. These methods typically lead to high polydispersity core–shell microcapsules [1] [2]. There is also limited control over morphology, poor reproducibility and compatibility with high encapsulation efficiencies.
Double emulsion using the RayDrop
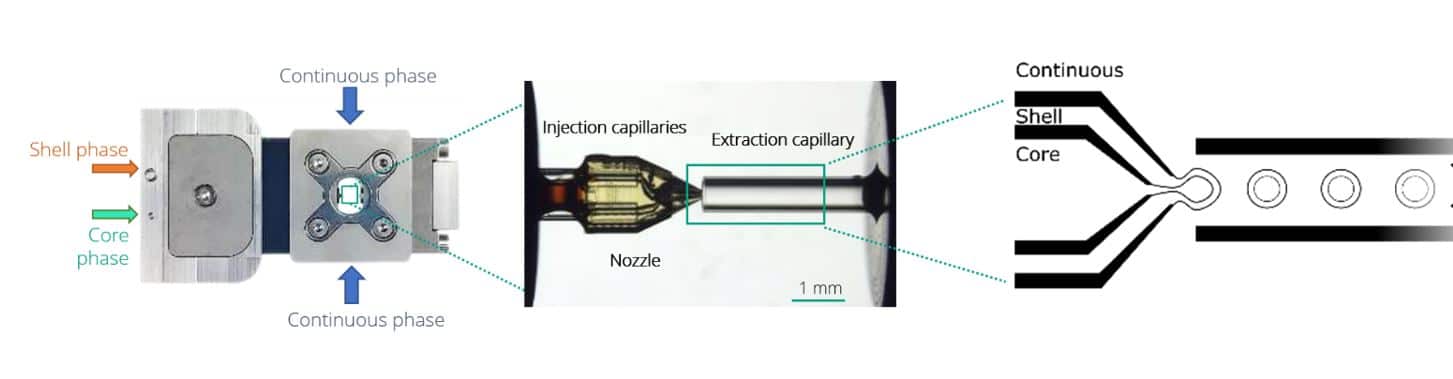
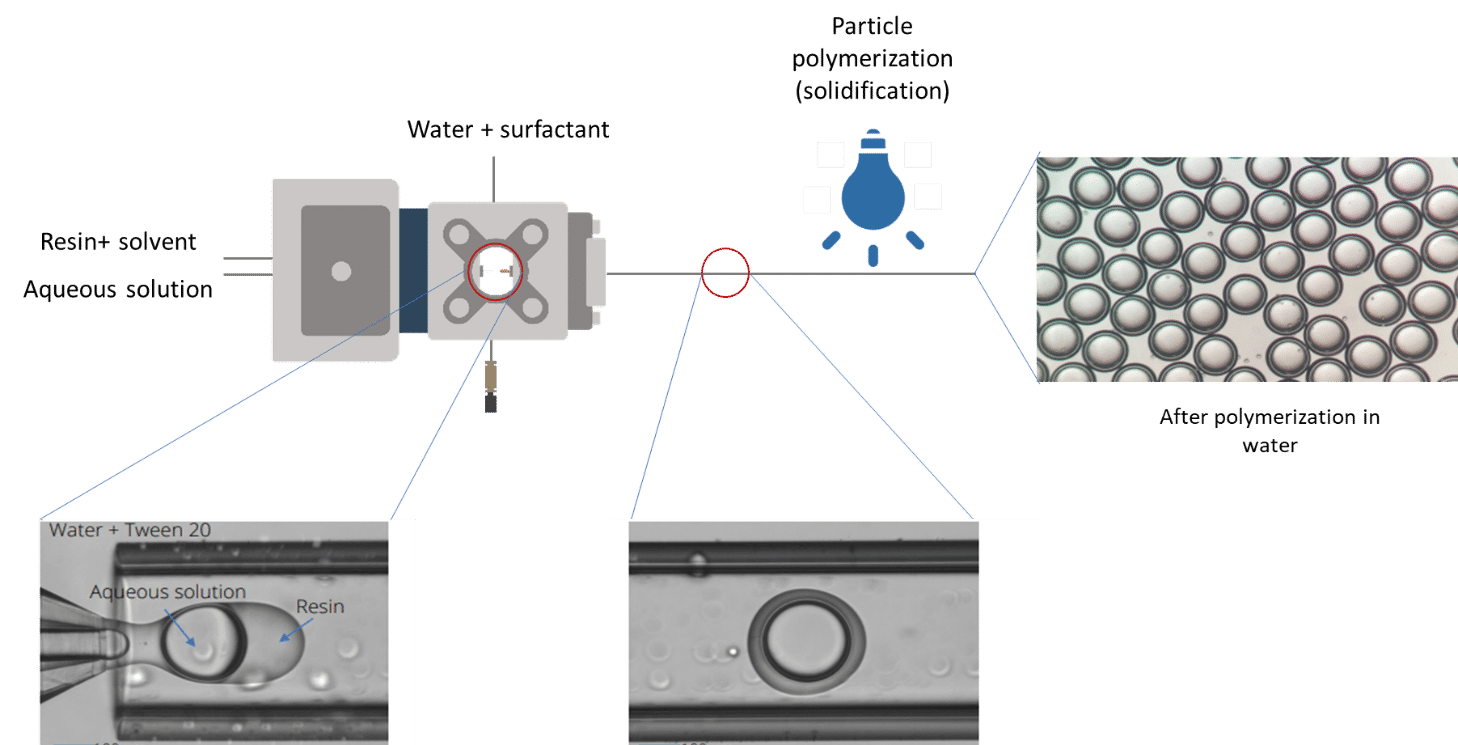
However, to obtain a highly reproducible UV-crosslinking of microcapsules, highly stable production must be achieved. Double Emulsions generated using microfluidic devices have been shown to overcome the difficulties of conventional methods by enabling fine control of the capsule size and shell thickness, while producing highly monodispersed, perfectly spherical microparticles. Among these devices, the RayDrop is the first to allow an easy to set up and robust production of double emulsion. It doesn’t rely on double coating treatment of PDMS/glass in planar chip [3][4] or the alignment of two round capillaries in a third square tubing, as in lab-made glass capillary microfluidic device [5] [6].As described on Figure 1, the Raydrop relies on the use of a 3D-printed injection nozzle carrying two fluids, the core and the shell phases. This is positioned in front of an extraction capillary in a cavity filled with the third (continuous) phase.
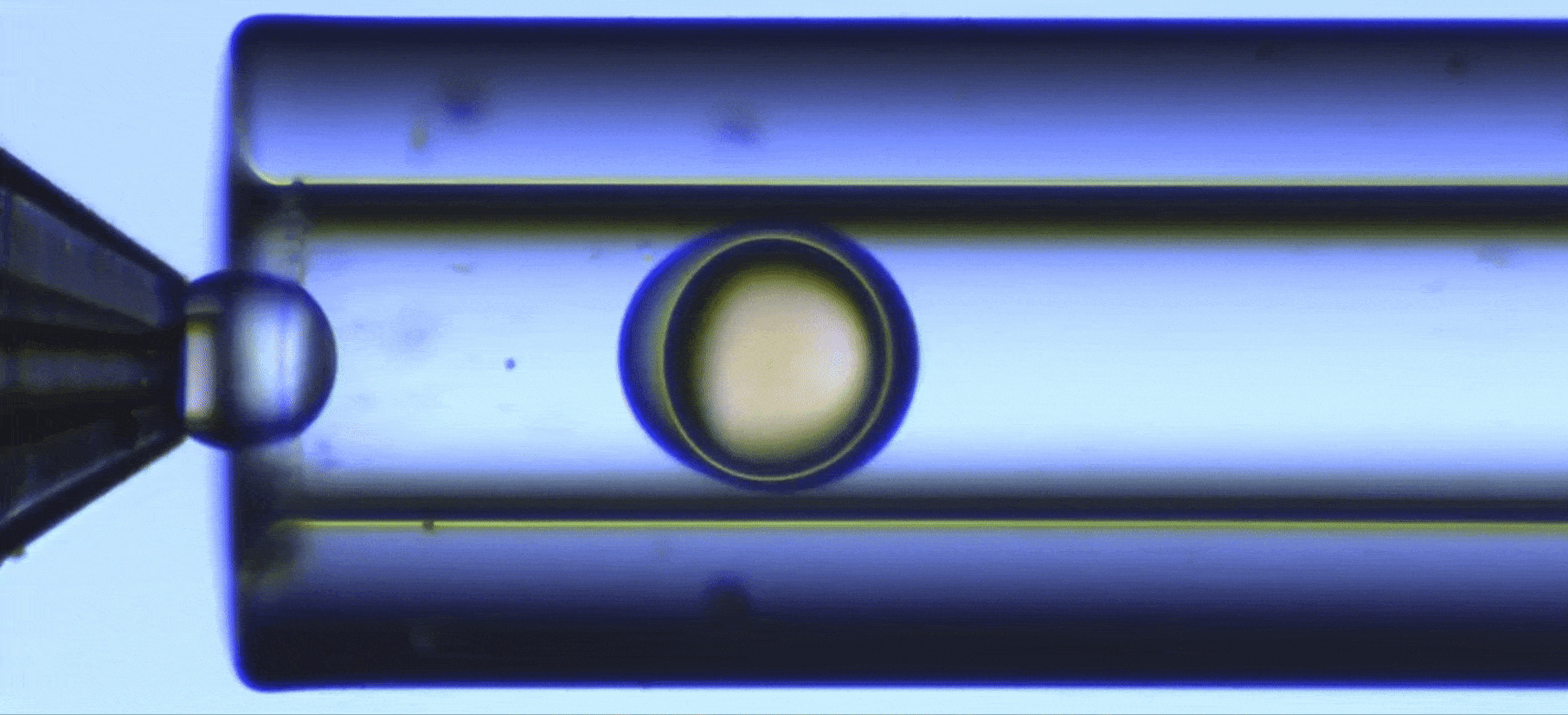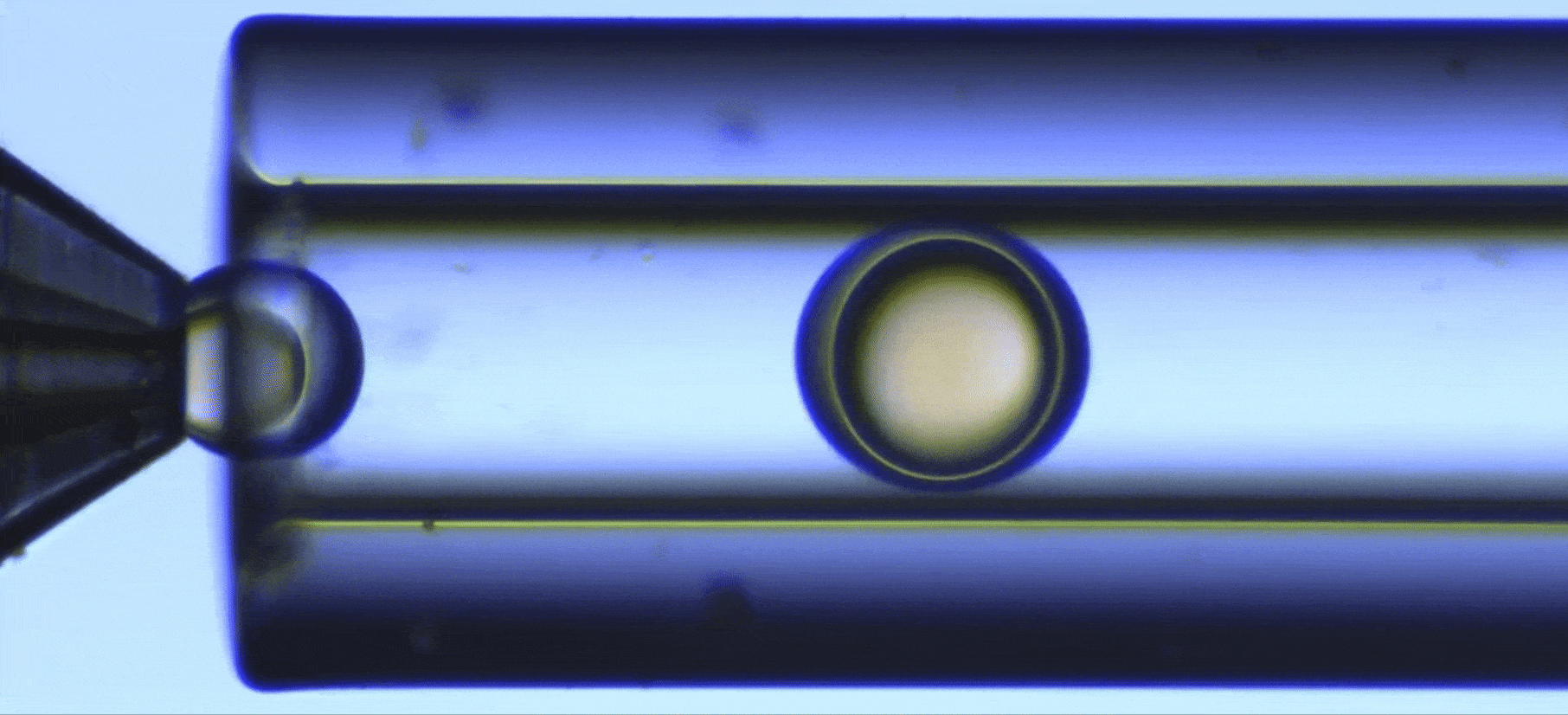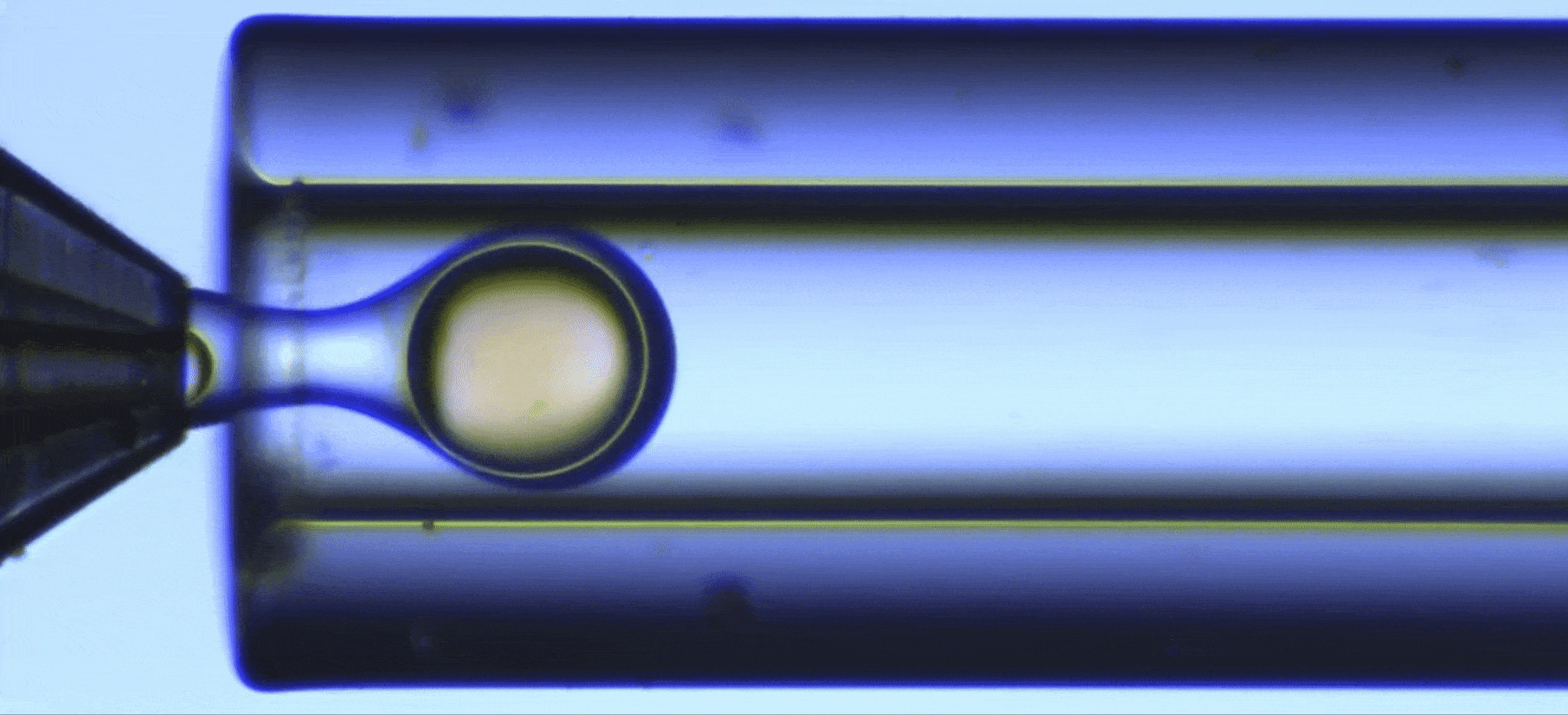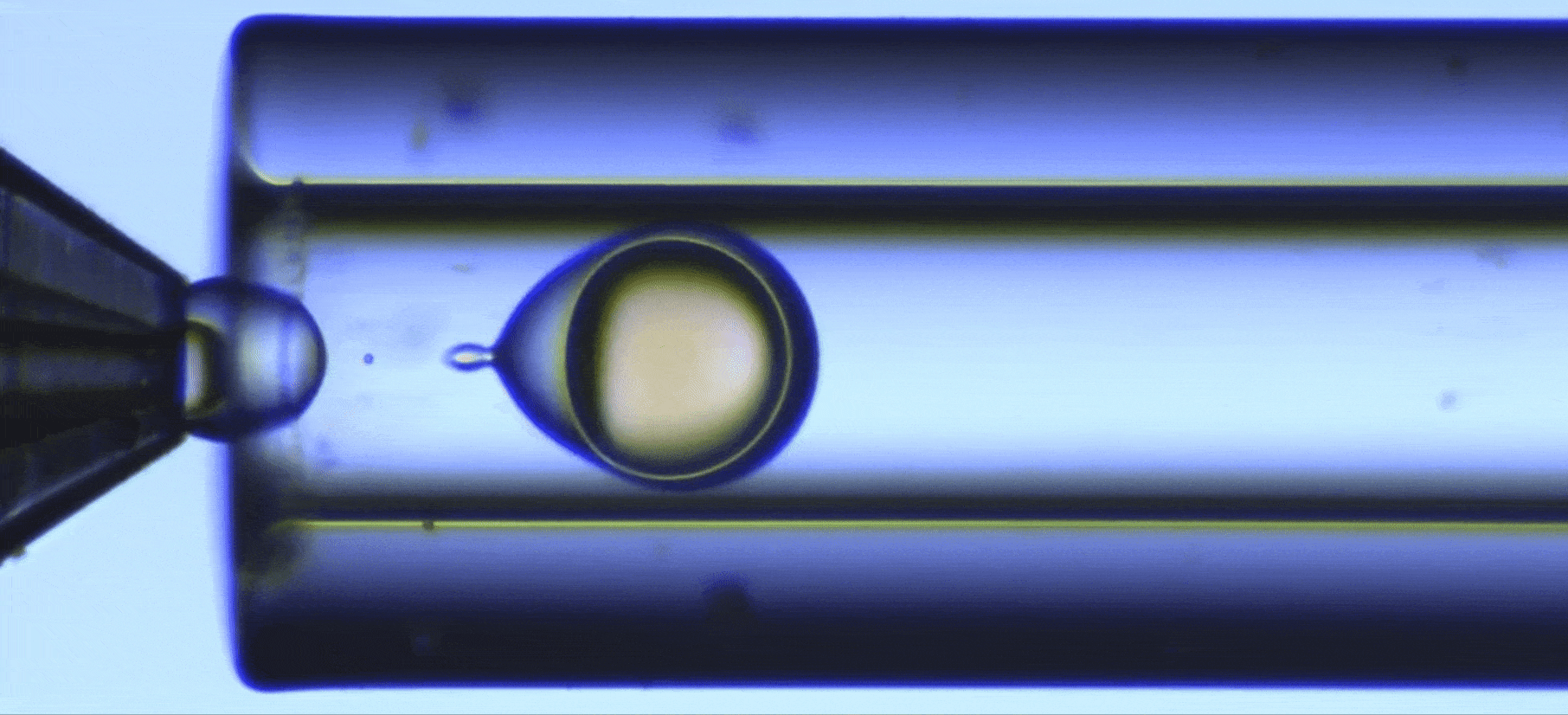
Perform a ultraviolet crosslinking of microcapsules
Microfluidic System for micro-sized capsules production
Reagents
Continuous phase and liquid for spacing:
- Distilled water with 1% Tween 20
DISPERSED PHASE
- Solution of commercial Allnex methacrylate-based resin with 0.1%
- 20% Ethyl acetate
- Photoinitiator Diphenyl (2,4,6-trimethylbenzoyl)phosphine oxide (TPO) from Sigma-Aldrich
UV-crosslinking of micro-sized capsules: Experimental procedure
Monodisperse polymer microcapsule synthesis is performed in 3 main steps:
- Generation of monodisperse droplet
- Droplet spacing
- Droplet solidification by UV irradiation
Droplet generation
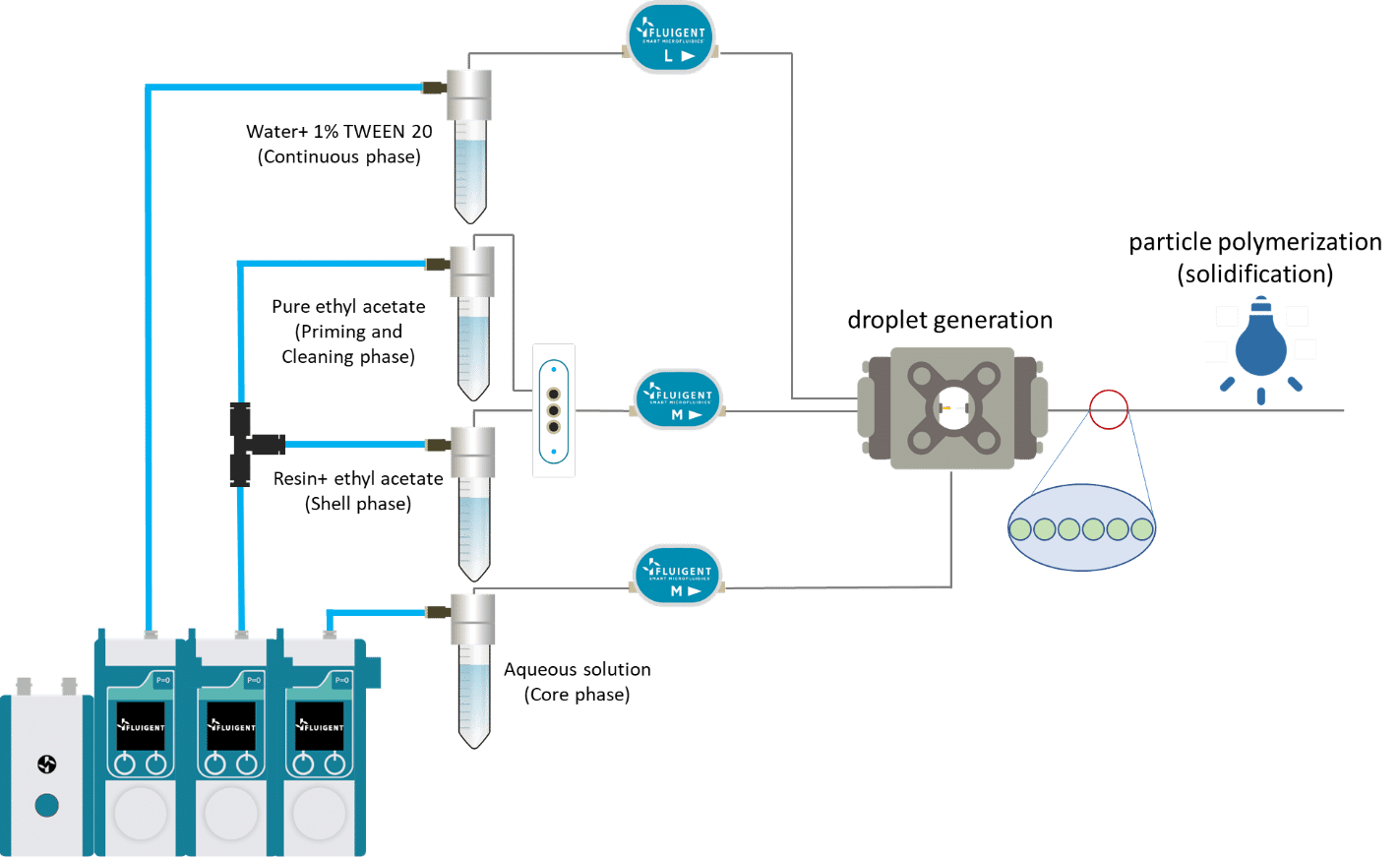
In this UV-crosslinking of microcapsules process, double emulsions are formed by pumping the three fluids through the Raydrop using a pressure controller. The flowrates are monitored using flowmeters.
Using the double emulsion Raydrop controlled double emulsification is achieved by dripping or jetting the core fluid into an immiscible shell fluid, which is then encapsulated by the third fluid. Core and continuous phases are aqueous phases, each being non-miscible with the shell.
As the core phase, deionized water is used and for the continuous phase, 1% wt aqueous solution of Tween 20. For the shell phase, a solution of commercial Allnex methacrylate-based resin with 0.1% wt of photoinitiator Diphenyl (2,4,6-trimethylbenzoyl)phosphine oxide (TPO) from Sigma-Aldrich and 20% ethyl acetate is used.
As shown on the scheme in Figure 3, reservoirs of resin solution and pure ethyl acetate are each connected to a valve. Ethyl acetate is first used as shell phase to initiate the double emulsion. When the system is stabilized, the valve is switched to the resin polymer. At the end of the process, ethyl acetate is flushed again to rinse the nozzle and tubing. The flushing of reactive or non-miscible species after an experiment is key to maintaining good operation of the system day after day. Fine control of the fluid flows leads to defined capsule and shell dimensions.
Droplet spacing
Once generated, droplets exit the Raydrop device with decreased flow velocity (due to the difference between the inner diameter of the downstream channel of the Raydrop and the exit tubing). To avoid clogging during the UV-crosslinking of microcapsules, increasing the spacing between droplets is highly recommended. Spacing between droplets can be performed by injecting liquid into the outer tubing in a co-flow manner.
Droplet polymerization: microcapsule solidification
After being spaced, the droplets are exposed to UV light. In-situ polymerization is achieved, meaning that the droplets are exposed to uv-light while still being moving forward in the output tubing connected to the Raydrop. Hard shell microcapsules are directly collected in the collection vial. The in-situ process avoids coalescence and deformation of the droplets that can arise in an ex-situ process where the droplets are polymerized after collection.
Figure 4: Setup description for UV-crosslinking of microcapsules process
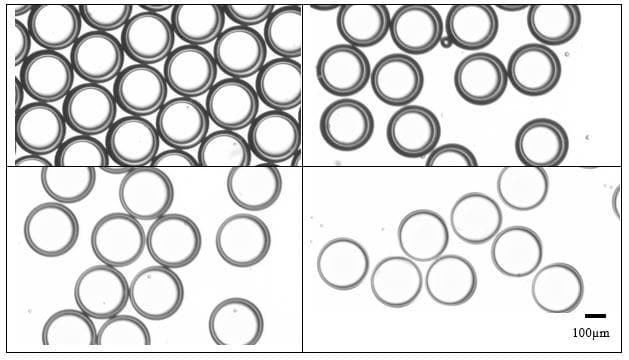
Result: Controlling capsule and shell dimensions by ajdusting the flowrate
In this method of UV crosslinking of microcapsules, the outer diameter of the emulsions and thus the capsule size can be selected within a broad range by changing the size of the collector capillary and the nozzle tip dimensions. This is easily achieved by a change of the two inserts.
For a configuration with the nozzle and output capillaries (respectively 90µm and 450µm ), as presented in this note, adjusting the flowrates of the fluids allows for fine control of the capsule dimensions (see Figure 4). With this setup droplet from 200µm to 300µm can be easily produced.
The shell thickness of microcapsules can also be varied by changing the ratio of flowrates of the shell and core phases, as shown in Figure 4. Here shell thickness varies from 10µm to 50µm.
Conclusion
In this application note we have demonstrated how to perform UV-crosslinking of microcapsules in a controlled and reproducible manner.
From droplet production to microcapsule polymerization every step is presented to allow a good reproduction of the experience. This system allows for production of highly monodisperse microcapsule (CV<2%) without interruption as compared to other methods used for microcapsule synthesis.
References
[1] Chen, P. W., Erb, R. M., & Studart, A. R. (2012). Designer polymer-based microcapsules made using microfluidics. Langmuir, 28(1), 144–152. https://doi.org/10.1021/la203088u
[2] Galogahi, F. M., Zhu, Y., An, H., & Nguyen, N. T. (2020). Core-shell microparticles: Generation approaches and applications. Journal of Science: Advanced Materials and Devices, 5(4), 417–435. https://doi.org/10.1016/j.jsamd.2020.09.001
[3] Abate, A. R., Thiele, J., & Weitz, D. A. (2011). One-step formation of multiple emulsions in microfluidics. Lab on a Chip, 11(2), 253–258. https://doi.org/10.1039/c0lc00236d
[4] Chong, D. T., Liu, X. S., Ma, H. J., Huang, G. Y., Han, Y. L., Cui, X. Y., … Xu, F. (2015). Advances in fabricating double-emulsion droplets and their biomedical applications. Microfluidics and Nanofluidics, 19(5), 1071–1090. https://doi.org/10.1007/s10404-015-1635-8
[5] Ekanem, E. E. (2015). Author ’ s Accepted Manuscript Double emulsion production in glass capillary microfluidic device : Parametric investigation of droplet generation behaviour. https://doi.org/10.1016/j.ces.2015.03.004
[6] Kim, S. H., Kim, J. W., Cho, J. C., & Weitz, D. A. (2011). Double-emulsion drops with ultra-thin shells for capsule templates. Lab on a Chip, 11(18), 3162–3166. https://doi.org/10.1039/c1lc20434c
Expertises & Resources
-
Expertise Addressing Air Bubble Issues in Microfluidic Systems Read more
-
Microfluidics White Papers Double emulsion for the generation of microcapsules – a Review Read more
-
Microfluidics White Papers Droplet-based Microfluidics – A Complete Guide Read more
-
Microfluidic Application Notes UV-Crosslinking of Microparticles Read more
-
Expert Reviews: Basics of Microfluidics Microfluidic Droplet Production Method Read more