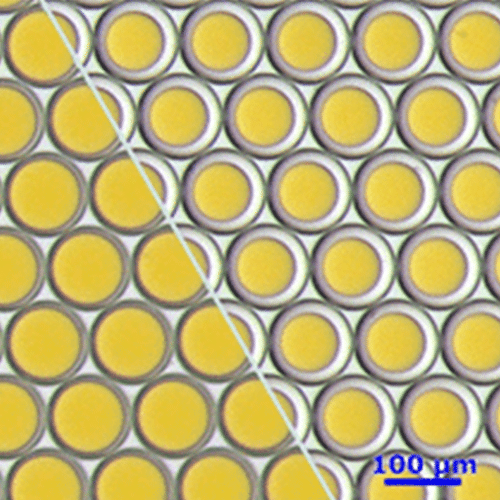
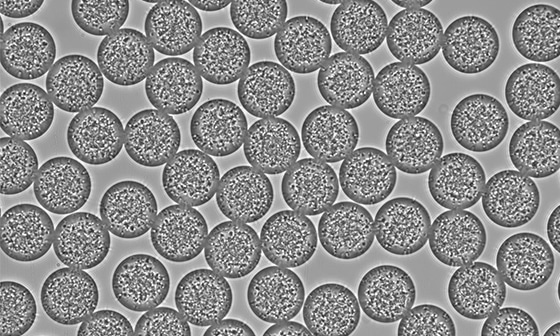
High monodispersity (2%)
PLGA Microparticle Production Pack (Automation Pack)
[O-SE-PLGAAP-PCK]A complete system for automated production of monodisperse PLGA microparticles
The PLGA microparticle production pack is a robust, high-performance solution to generate polymer microparticles in a homogenous and fully controlled manner.
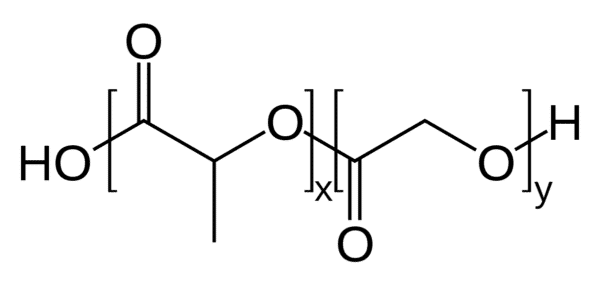
The performance brought by the RayDrop droplet generator, with the combination of Poly(lactic-co-glycolic acid) as an encapsulation polymer, and ethyl acetate as a solvent, provides a biocompatible solution lowering both hazard risk and precipitation time.
Suitable for biological applications, the RayDrop and the PLGA Microparticle Production system offer an automated solution for one of the most successful drug delivery systems in laboratories and clinics. The control and generation of droplets allow for highly monodisperse and continuous production compared to batch emulsion methods.
Therefore, the application of droplet-based microfluidics for the production of PLGA microparticles is a powerful tool that allows us to obtain particles with high monodispersity

- Repeatability
- Automated
Continuous production
- Adaptable
Control on particle size
Features of PLGA Microparticle Pack
A complete PLGA Microparticle Production system
With this package, you have all the components necessary to start generating PLGA microparticles.
An engineered solution
We built this PLGA Microparticle Production Pack with the right pressure controllers, microfluidic chips, and valves to make sure you have the highest flexibility in terms of droplet size and generation rate.
A dedicated protocol
A protocol is available to assist you setting up and starting your experiments
Customization possible
We can adapt the package to fulfill your needs (droplet size, generation rate)
How to produce PLGA microparticle with our Pack?
In our Application Note, we demonstrate to you how the system for the production of PLGA Microparticle creates excellent reproducibility and significantly improves monodispersity (CV < 2%) as compared to other methods on the market. It This automated PLGA microparticule production technology brings together all the benefits of pressure-based solutions and droplet microfluidics to focus on the experiment, and even offers the chance to automate protocols for producing different microparticle sizes.
Main products of the PLGA Microparticle Production System
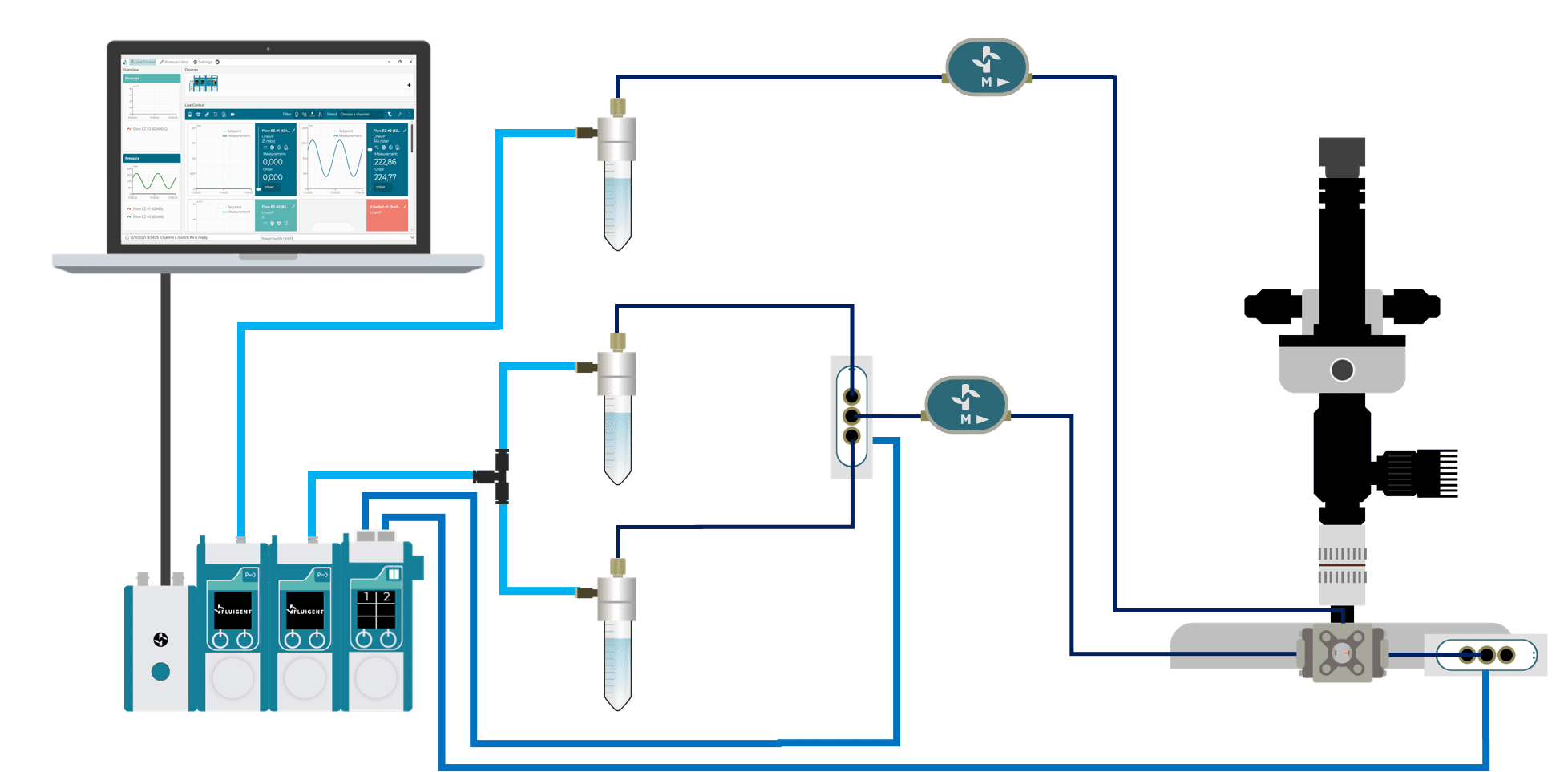
The PLGA Microparticle Production Pack is made up of various Fluigent microfluidic devices, which are listed below:
Two different pressure controllers (Flow EZ) are used to handle fluids.
A 3/2 valve (2-SWITCH) is used to switch between pure ethyl acetate and PLGA dissolved in ethyl acetate and a second valve is also used in particle production output to switch between a waste and recover the sample.
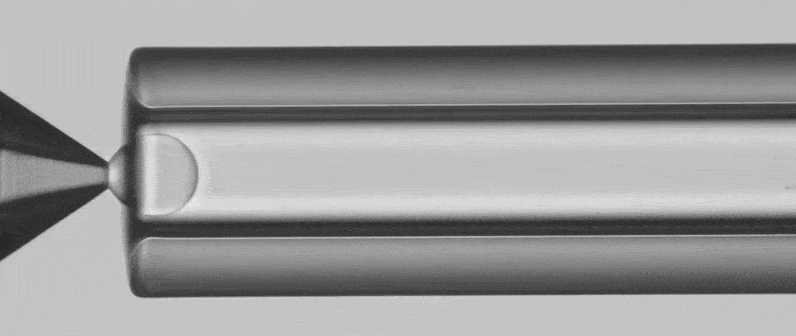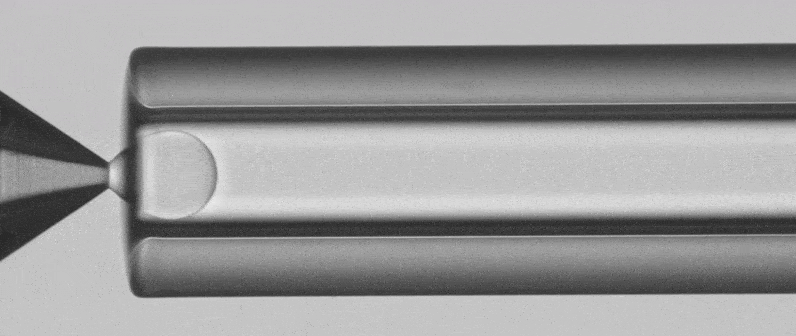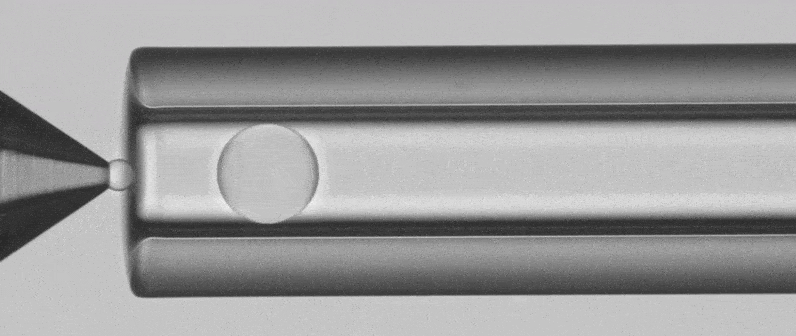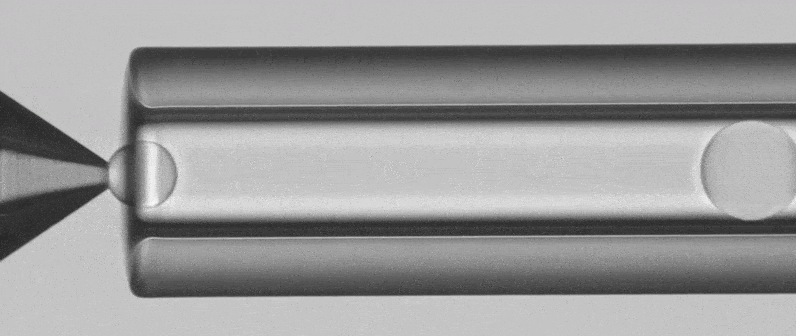
Two Flow Unit sensors are used in the PLGA Microparticle Production Package to monitor and control the internal and external phases flow rates during the run all. The RayDrop Single Emulsion is used to generate PLGA microparticles. It relies on the alignment of two glass capillaries inside a pressurized chamber. A 3D-printed micro-nozzle is additionally connected at the tip of the injection capillary, enforcing the dripping (fluids flow at low rates) of small droplets. This non-embedded design presents both the characteristics of a co-flow and a flow focusing, and is thereby called non-embedded co-flow focusing. The nozzle and outlet capillary are aligned in the continuous phase chamber, the dispersed phase comes through the nozzle to create the microparticles into the continuous phase and exit by the outlet insert.

Microfluidic Single Emulsion Device
RayDrop Single Emulsion

Microfluidic flow controller
Flow EZ™

Bidirectional Microfluidic Flow Sensor
FLOW UNIT | FLOW UNIT +

Microfluidic Sampling Valve
2-SWITCH™ 3-port/2-way

Microfluidic valve controller for flow redirection
SWITCH EZ

Airtight metal tube caps for microfluidics
P-CAP series

Microfluidic Software Control
Microfluidic Software control

Real-Time Control & Lab Automation Software
OxyGEN – The new way to get full control of your microfluidic system and automate your lab setup.
Webinar – Drug encapsulation in biocompatible microparticles for drug delivery
In pharmaceutical area, Active Pharmaceutical Ingredient (API) encapsulation into biodegradable and biocompatible polymers is widely used for producing new smart Drug Delivery Systems. The goal of these drug delivery systems is to supply doses of drugs for a sustained period of time at targeting specific sites in the body. This can be achieved by efficiently loading drugs into microparticles which will protect the API during physiological transport and release it when the microparticles have reached their specified target location(s). To regulate the release of the drug, it is critical to controllably produce microparticles with known sizes and a homogeneous size distribution as the rate of drug release is proportional to microparticle size.
Among all techniques available for microparticle production, microfluidic and droplet-based microfluidic ones appear as the best solutions to precisely control microparticle production in terms of size, API loading and encapsulation efficiency.
In this webinar, we discuss how microfluidics can allow API encapsulation into biocompatible and biodegradable microparticles for drug delivery.
What you’ll learn:
- How microfluidics can be used for API encapsulation in microparticles?
- What is droplet microfluidics?
- Learn a new method for microparticle production
Conference animated by :
- Adam Meziane, Product manager, Fluigent
- Adrien Dewandre, Technology Lead, Secoya Technologies
- Arnaud REITZ, R&D project manager, Fluigent
April 30th 2020
Specifications
PARTICLE PRODUCTION
| Dispersed phase | PLGA lactide: glycolide (75:25), mol wt 66,000-107,000 |
| PLGA concentration used | 2%, 5% ans 10% |
| Continuous phase | Ethyl acetate |
| Doplet size range | 60µm to 120 µm |
| Particle size range | 20µm to 50 µm |
| Production rate | Up to 60mg/h |
| Production frequency | U to 1000Hz |
| Monodisperity | 2% |
FLOW CONTROL
| Pumps | Fluigent Flow EZ™ (2 bar) |
| Flow sensors | Fluigent FLOW UNIT (M and L) |
| Automated valves | Fluigent 2-SWITCH™ |
IMAGING
| Microscope | Fluigent Digital high-speed microscope |
OxyGEN
| Control in real-time, protocol automation, data record and export |
| ver. 2.2.0.0 or more recent |
Imaging
| OptoViewer Software |
| Traditional methods (Batch methods) | Fluigent PLGA microparticle production station | |
| Particle size distribution | ~20% | ~2% |
| Reproducibility | Low | High |
| API mixing | Uneven | Uniform |
| Live particle size control | No | Precise |
| Continuous / In line production | No | Yes |
| Microfluidic methods available on the market | Fluigent PLGA microparticle production station | |
| Particle size distribution | ~5% | ~2% |
| Semi automated production | No | Yes |
| Ethyl acetate dedicated protocol | No | Yes |
| Device regeneration | No (glass chip changed when clogged) | Yes (the RayDrop can be maintained) |
| Connectors | Non standard, user dependant quality (leakage, blockage) | Standard fittings for better sealing |
Can I use a polymer other than PLGA?
The method has been designed for PLGA. Changing polymers may change the physical fluid properties and lead to different results.
How can I prevent the clogging from PLGA?
Follow the procedure detailed in the application note. Please note the priming and cleaning procedures.
The nozzle (or capillary) sems clogged with PLGA. How can I fix it?
If the usual cleaning procedures from the good practice guide cannot unclog the nozzle, contact customer support for help.
Some of the ethyl acetate has flowed into the continuous phase chamber. What should I do?
If some ethylacetate without PLGA has flowed into the chamber it can be flushed out easily.
Follow the instructions in the good practice guide.
I can see a drop of ethlacetate fixed on the outside of the nozzle. How can I get ride of it?
If some ethylacetate without PLGA is fixed around the nozzle, it can be easily flushed out.
Follow the instructions in the good practice guide.
Some of the PLGA solution has flowed into the chamber. What shoulg I do?
If some PLGA gets into the chamber, it has to be eliminated quickly.
Refer to the good practice guide of the PLGA Microparticles Production Pack for the exact procedure.
Air bubbles regularly appear during the experiment. What should I do?
If air bubbles appear in the system for the production of PLGA Microparticle during experiment we advise one to switch to the ethyl acetate solution (if you are in the PLGA configuration) and let it flow for a minute to remove all PLGA from the system.
Then make sure that all connectors are tightened properly.
Refer to the application note procedure to avoid air bubble infiltration.
Expertise & resources
-
Microfluidics White Papers Double emulsion for the generation of microcapsules – a Review Read more
-
Fluigent products manual Good practice guide PLGA station Download
-
Microfluidics White Papers Droplet-based Microfluidics – A Complete Guide Read more
-
Fluigent Products Datasheets PLGA Microparticle production station Datasheet Download
-
Fluigent Products Datasheets Raydrop single emulsion datasheet Download
-
Expert Reviews: Basics of Microfluidics The Raydrop | A new droplet generation device based on non-embedded co-flow-focusing Read more
-
Microfluidic Application Notes PLGA Microparticles Synthesis Read more
-
Fluigent Products Datasheets FLOW UNIT Datasheet Download
-
Fluigent Products Datasheets Flow EZ™ Datasheet Download
Related products

PLGA Microparticle Production Standard Pack
PLGA Microparticle Production Pack (Standard Pack)
See the offer
Microfluidic Single Emulsion Device
RayDrop Single Emulsion
See the offer
Bidirectional Microfluidic Flow Sensor
FLOW UNIT | FLOW UNIT +
See the offer
Microfluidic flow controller
Flow EZ™
See the offer
Microfluidic valve controller for flow redirection
SWITCH EZ
See the offer
Real-Time Control & Lab Automation Software
OxyGEN – The new way to get full control of your microfluidic system and automate your lab setup.
See the offer