Optimizing Microfluidic Perfusion: Best Practices and Innovations
The importance of perfusion and pressure control in Organ-on-Chip (OOC) technologies. It compares various pump and flow control systems, outlines key factors to consider for optimal bubble-free perfusion and showcases real-world examples of OOC applications using Fluigent devices.
What Is Microfluidic Perfusion
To define the term microfluidic perfusion and distinguish it from general continuous flow:
Perfusion typically refers to the controlled delivery of fluid through a structure, such as tissues or channels—a term predominantly rooted in medical terminology. Perfusion in microfluidics denotes the continuous flow of fluid through microscale channels, often in reference to tissues or reaction zones. [1][2]
With the continuous evolution of the field, microfluidic perfusion has established a critical role in live-cell analysis, organ-on-a-chip platforms, and high-sensitivity analytical assays such as single-molecule detection. Whether implemented as continuous, multiplexed, or sequential perfusion, it is essential to be able to precisely control flow dynamics—such as flow rate, laminar versus turbulent flow, and directionality.
Table 1: Relevance of Microfluidic Perfusion in Life-Science Research
| Field of application | Usage | To explore further |
|---|---|---|
| Organ-on-a-chip/ Dynamic Cell Culture | Continuous perfusion of media to replicate physiologically relevant flow conditions, including shear stress, nutrient and oxygen exchange, and waste removal, to model in vivo microenvironments. | 1. HUVECs cultures under shear stress 2. Controlling shear stress in 3D cell culture 3. Multiplexed blood vessel-on-a-chip |
| Live-cell imaging / Long-term Microscopy | Delivery of nutrients and reagents during imaging sessions to maintain cell viability and enable real-time observation of cellular processes. | 1. Automating calcium imaging in neural cells 2. Automated multiplexed immunolabelling with Aria |
| Drug response testing | Dynamic administration and sampling of pharmacological agents to assess dose-dependent cellular responses, facilitating high-throughput screening and toxicity evaluation. | 1. Tongue on chip : sweet and bitter taste response 2. Cancer-on-chip treatment response |
| Perfusion bioreactors and cell sorting | Scaled-up systems employing continuous perfusion to manufacture bioreagent (cells, DNA, exosome) and to sort and qualify it. | 1. Cell sorting microfluidic platform |
In organ-on-a-chip applications, this creates an in vivo–like microenvironment compared to static cultures. Perfusion provides a sterile, stable environment by avoiding repeated manual interventions and environmental fluctuations. The resulting stability enables long-term experiments, including live-cell imaging, monitoring of calcium transporters, and drug testing. Perfused systems enhance cell viability over extended periods and enable precise, dose-controlled exposure to multiple compounds.
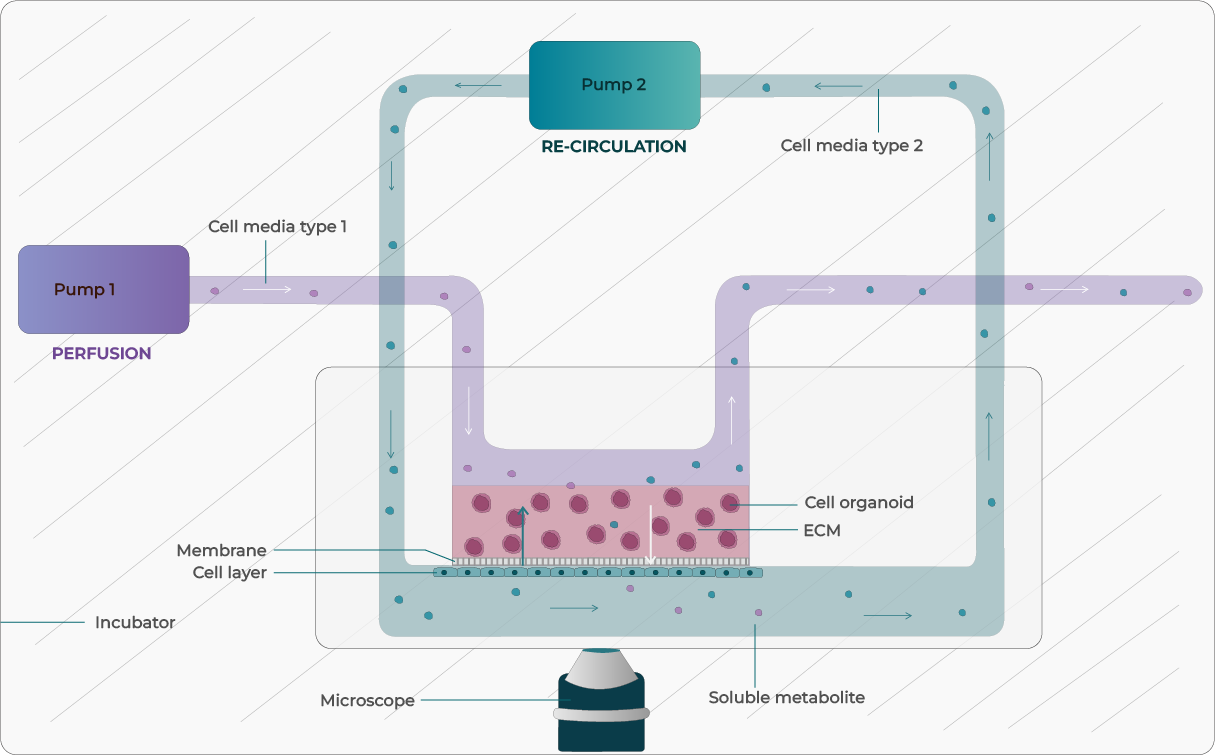
Figure 1: Illustration of Microfluidic Perfusion (open loop and closed loop e.i. recirculation) in OOC System
Best Practices in Microfluidic Perfusion
Effective perfusion relies on the integration of suitable materials, precise channel architecture, and design strategies that support the biological or chemical application.
Selecting Materials Based on Application Requirements
Depending on the application, the choice of microfluidic chip materials is influenced by variables like mechanical qualities, chemical compatibility, and gas permeability which can be tailored to the specific application. These choices are guided by research and the targeted biological modelling.
Various microfluidic chip materials:
- PDMS (Polydimethylsiloxane): It is chosen for oxygenation purposes due to its gas permeability, however it can absorb small hydrophobic molecules, which may interfere with drug toxicology assays [3].
- Glass and thermoplastics (e.g., PMMA, COC): These materials are often chemically inert with low adsorption and preferred for applications requiring minimal leaching.
- Hydrogels: Emerging as biocompatible scaffolds for fully soft microfluidic systems, particularly for tissue-mimetic and 3D culture environments [4].
Each tissue in the human body varies in function and morphology, therefore biomimetic models should account for this difference. For instance, materials suitable for mimicking bone (Young’s modulus ~20 GPa) will differ significantly from those used for brain tissue (Young’s modulus ~2 kPa) [5][6]. For hypoxic environments, glass or low-permeability thermoplastics are preferred.Soft tissue modeling is better suited to flexible membranes or hydrogels.
In addition to material consideration, there are also modifications that can be made to the internal surfaces to support the intended biological function:
- Enhanced cell adhesion, coat with ECM proteins (e.g., fibronectin, collagen).
- Non-specific adsorption prevention, apply PEGylation, BSA coating or other antifouling coatings [7]
- Plasma oxidation or chemical functionalization can improve hydrophilicity and wetting behavior.
Optimizing Chip Geometries and Dimensions
Channel design has an impact on fluid dynamics and cellular responses within microfluidic systems. The microenvironment’s architecture affects the mechanical cues received by cells [8], and channel dimensions and volumes impact reagent diffusion and subsequent biological interactions.
- Shear stress influences key cellular responses including morphology, migration, and gene expression. Flow conditions should consider channel geometry and flow rate: to approximate the shear stress to the in vivo-like shear ranges (typically 1–20 dyn/cm²) depending on the cell type.
- Perfused channel dimensions affect reagent diffusion and sample exposure time. While diffusion contributes to the transport of molecules within microfluidic systems, the dominant factor influencing cellular response in perfusion assays is the convective flow of reagents [9]. Precise control over channel dimensions and flow rates ensures that cells are exposed to well-defined reagent concentrations and temporal profiles. Variation in channel dimensions can alter flow velocity and residence time, leading to inconsistent reagent delivery or diffusion-dominated transport, which blurs concentration gradients and reduces assay reproducibility. Designing channels to optimize reagent perfusion enables reproducible cellular responses to stimuli, critical for quantitative biological assays.
- Proper sealing of the microfluidic device is essential to maintain controlled fluidic environments. Leaks caused by open ports, improperly bonded layers, or uncovered membranes can introduce unwanted flow paths, cross-contamination, or loss of reagents. This can disrupt fluid dynamics, therefore it is important to be able to detect and prevent leaks. Meticulous sealing—using appropriate adhesives, bonding techniques, or mechanical clamps—ensures device integrity, especially in multilayer or co-culture chips where spatial separation is vital. Proper sealing also facilitates sterile handling and reduces bubble formation by preventing air ingress.
! Note on Microfluidic Device Use:
When using commercially available microfluidic chips or flow cells, it’s essential to calibrate system settings and consider the optical, chemical, and physical properties of the materials. For instance, PDMS can absorb small hydrophobic molecules, potentially affecting dosing accuracy, while issues like membrane leakage or deformation in hydrogel-based chips can compromise sterility and sealing prior to perfusion.
Avoiding Bubbles to Ensure Homogenous Flow
Bubbles can be a source of disruption to continuous flow. In 3D cell culture they cause cell damage, in assay it interferes with homogeneity of the flow profiles.
- Consistent Temperature: Temperature fluctuations can cause dissolved gases to nucleate into bubbles; for example, a temperature increase of just a few degrees Celsius can reduce gas solubility enough to induce bubble formation [10].
- Bubble prevention strategies include degassing media before use and incorporate bubble traps or in-line degassing membranes to capture and remove air bubbles that can disrupt perfusion. Incorporating bubble traps or degassing membranes on-chip physically removes bubbles before they reach sensitive cell culture regions.
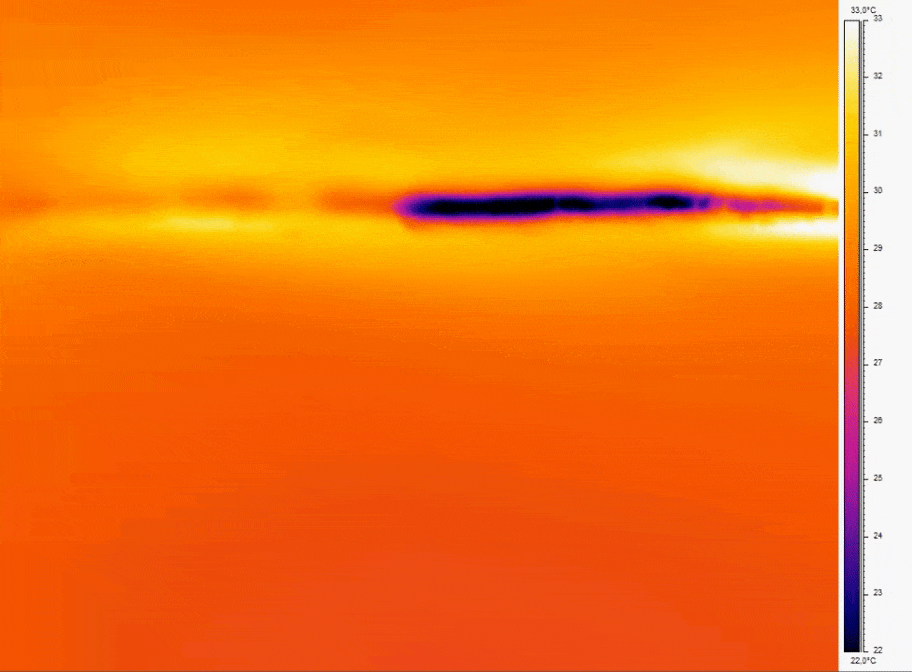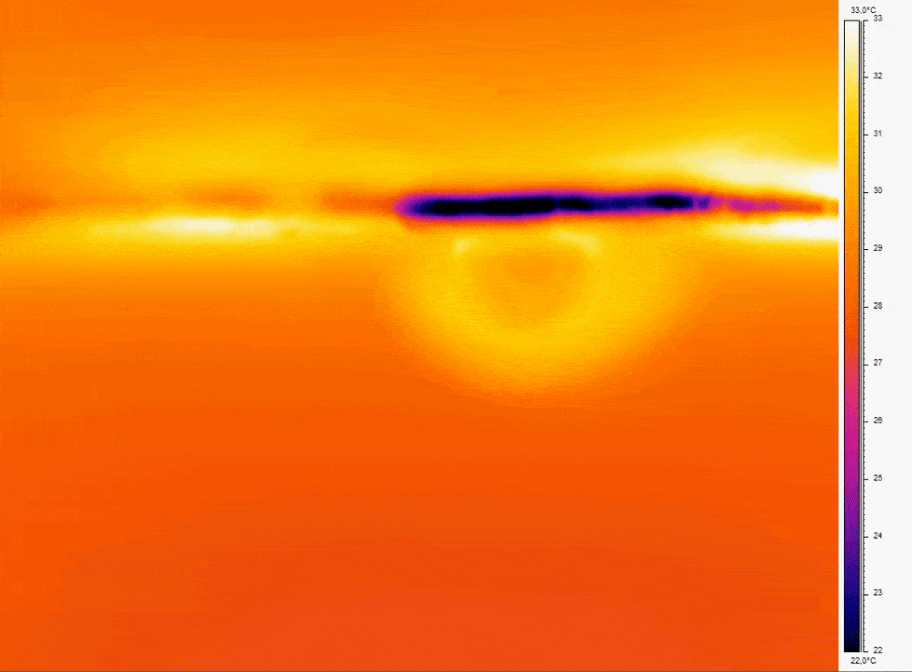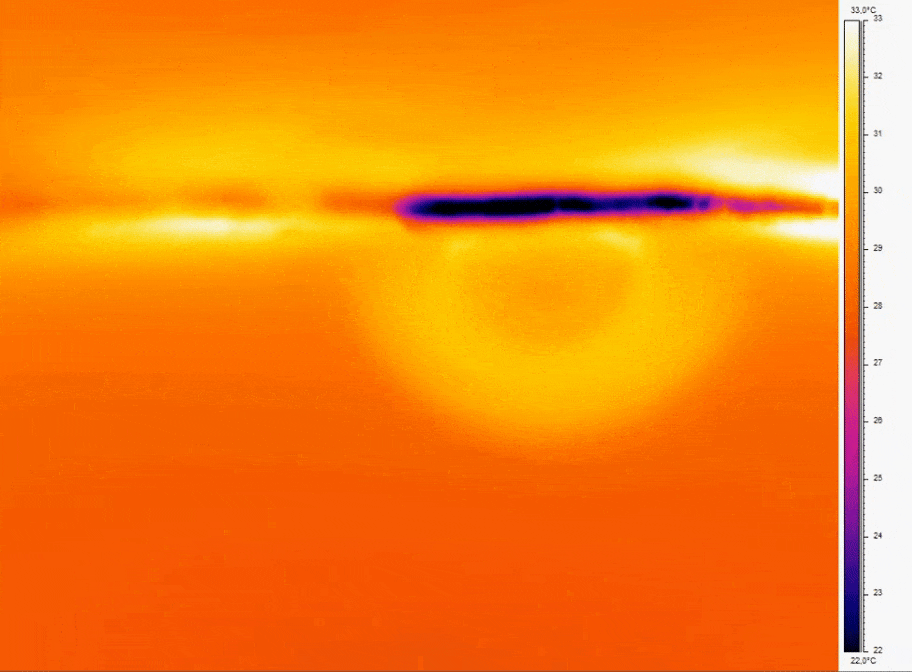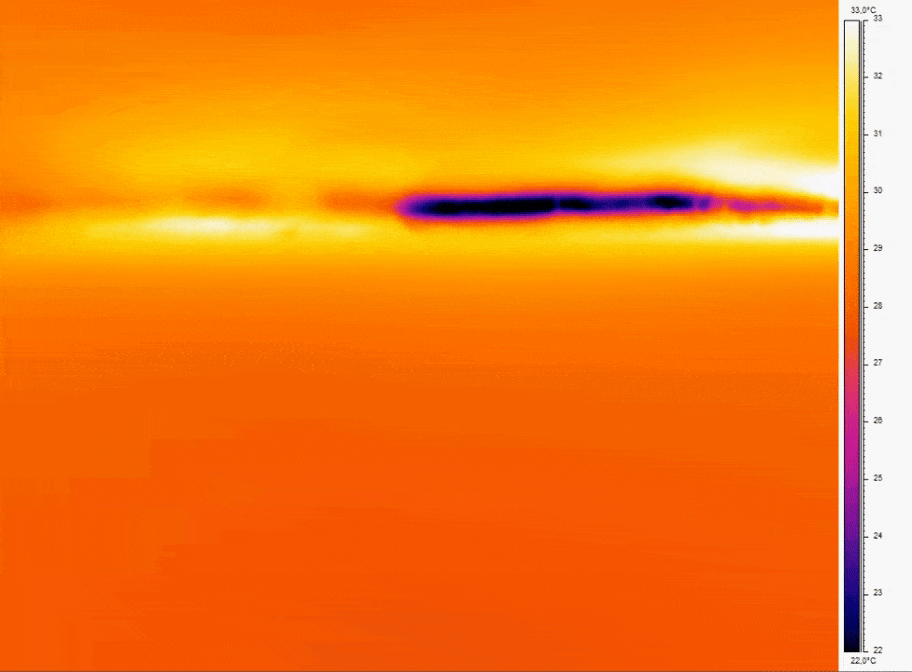
Figure 2: Thermal imaging of bubble nucleation in microfluidic channel
Selecting Appropriate Perfusion Pumps for Flow Control
Choosing the right pump type and flow control depends on the definition of technical specifications. There are various instruments that are used for microfluidic perfusion:
- Pressure-driven pumps provide precise, programmable flow profiles with rapid response times, making them ideal for sensitive applications requiring stable, low-pulsatility flow; this stability helps maintain laminar flow, critical for replicating in vivo shear stresses.
- Syringe pumps deliver constant flow rates with typical accuracy of around 0.25%, but manual refilling interrupts flow and introduces pulsatility, which may affect sensitive cells or reactions.
- Peristaltic pumps, although easy to use, inherently produce pulsatile flow that can stress cells and complicate quantitative assays. It significantly affects the cell survival, continue to read how it affects endothelial 3D cell culture.
Here you can find comprehensive review on the flow control technologies.
In addition to selecting the proper perfusion technology, it is also important to regularly calibrate and clean it. For example, biofilm deposits on flow sensors can cause drifts, even a small deviations in flow rate can significantly alter shear stress and cellular responses, impacting data reliability.
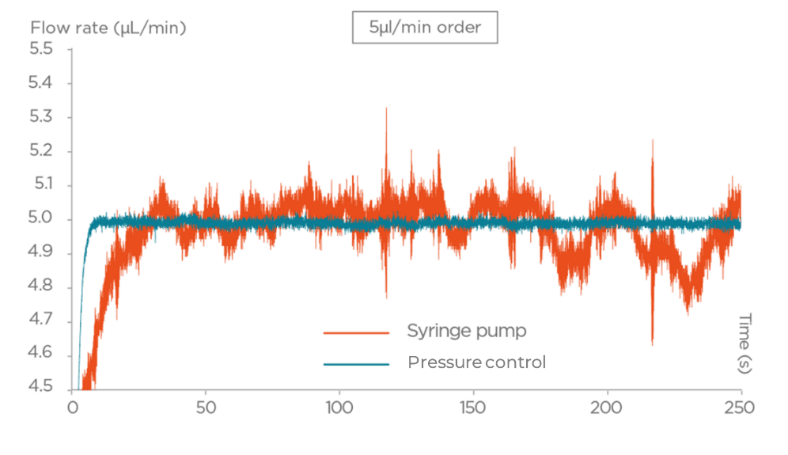
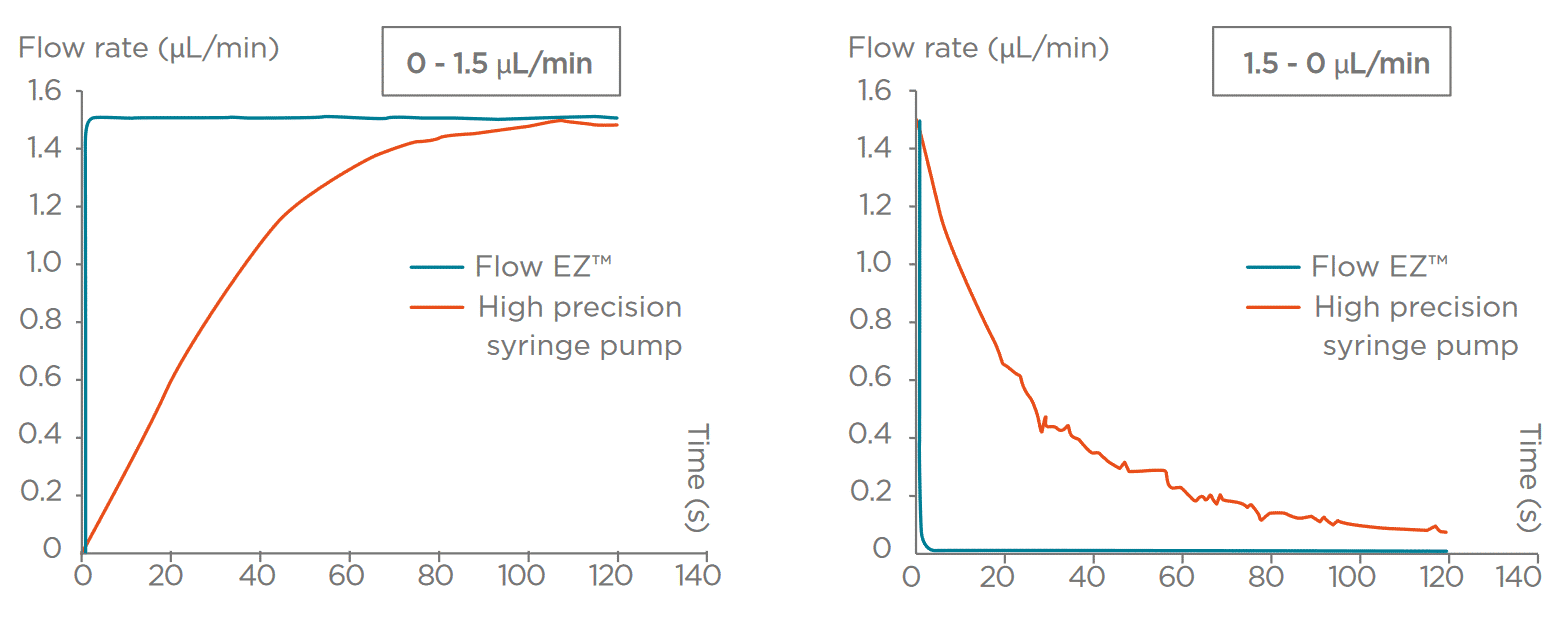
Figure 3: Performance of Pressure-driven flow controller vs Syringe Pump
Innovations in Microfluidic Continuous Flow
Automated Multiplexed Systems
Innovative approaches to multiplex the flow in microfluidics address the challenges of precisely managing multiple inputs and reagents and ensuring the stable liquid distribution in parallel channels. In pressure-driven microfluidics with ability to control multiple ports, allows creating specific manifolds allow parallel reagent delivery and medium recirculation.
To multiplex the sequential delivery of up to 10 reagents, Aria can be used to perfuse a chip with up to 10 distinct reagents, and its software allows automated injections.
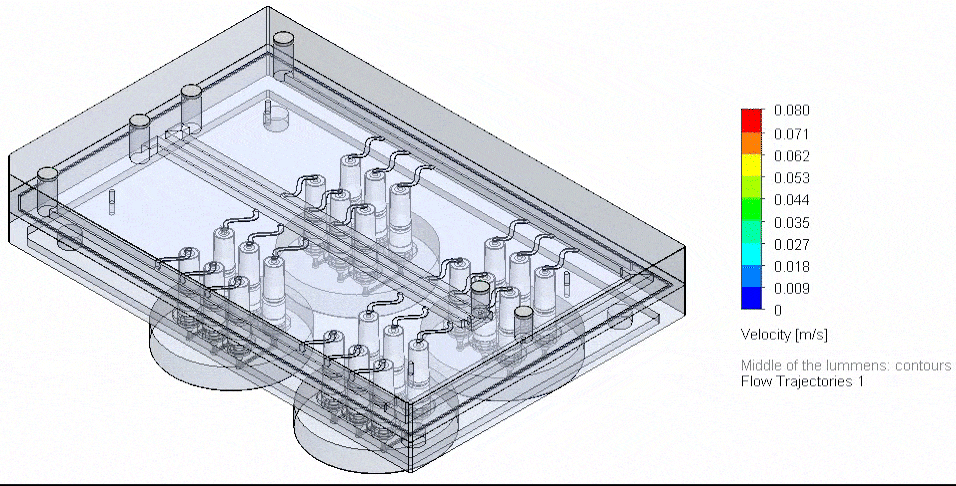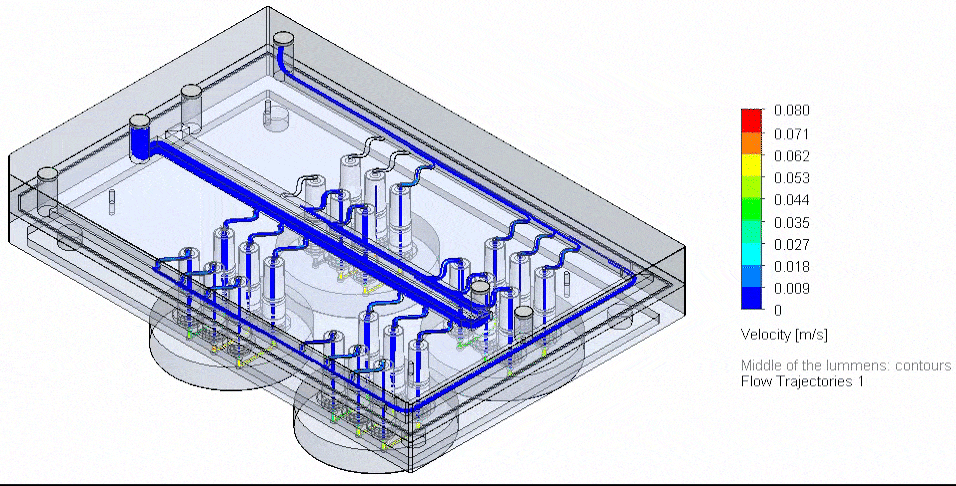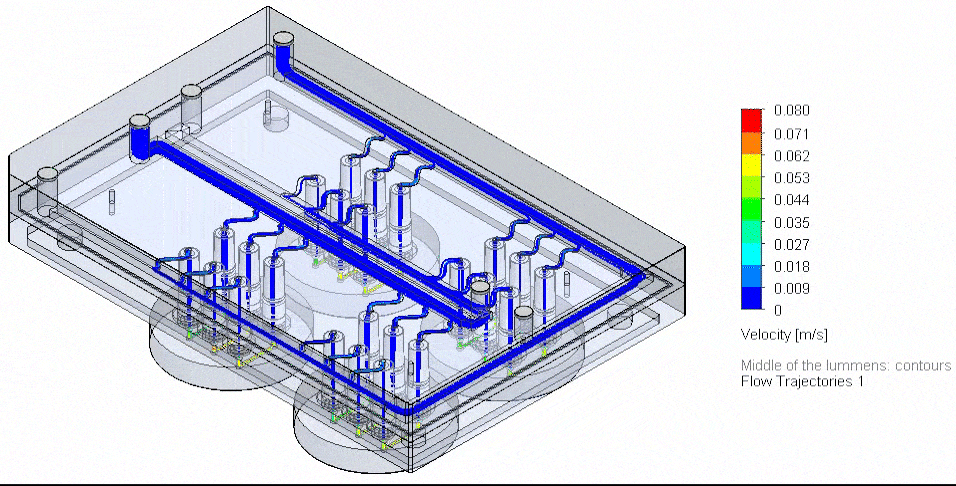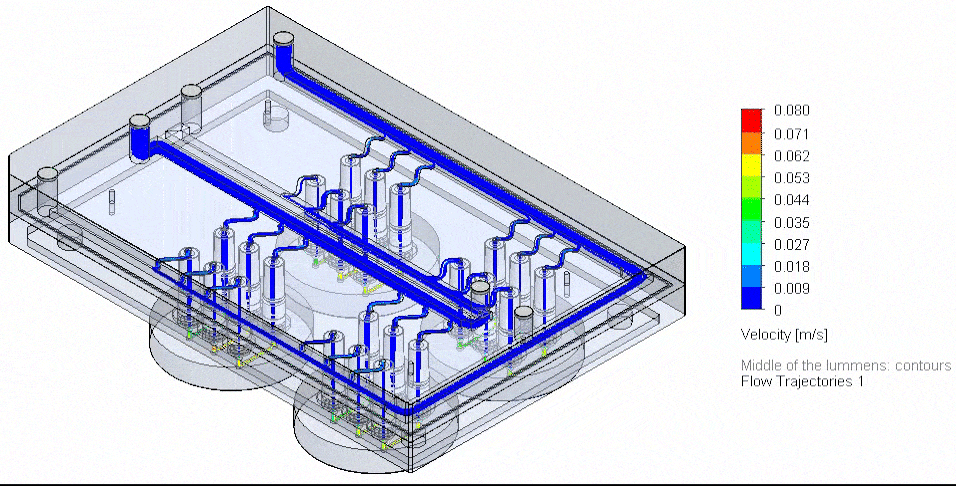
Figure 4: Flow Illustration of flow board automation from 3D-VoC model
Integrated Real-Time Sensing
On-chip and in-line sensor integrations (optical or electrochemical probes, or biosensors, for example for the inflammatory markers) are being used in the research to analyze and measure experimental outcomes. Closed loop control allows these sensors to continuously monitor the culture environment in real time and use the data to automatically adjust perfusion parameters. This ensures stable physiological conditions essential for cell health and reproducibility. One exampleis a compact microfluidic-embedded optical sensor array that was developed to track pH and dissolved oxygen in flowing media. This permits dynamic adjustments to maintain setpoint conditions during perfusion [11].
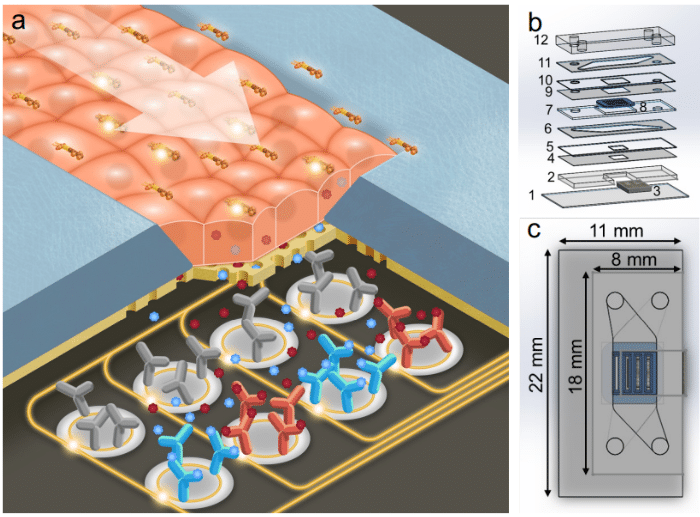
Figure 5: Photonic Sensor-Enabled Tissue Chip (a. Schematic of the working principle of the device. b. Exploded view with layers. c. Top view with outer dimensions of the device.
Organ-on-a-Chip Media Perfusion
With the growth of organ-on-a-chip adoption, there have been adaptations of the technology to be able to match the physiological requirements of the models. Close loop recirculation has been adapted to provide many benefits. ensures sterility, provideslong-term recirculation, reduces the cost of reagent, and can amplify the secretome of the interest.
Continuous microfluidic perfusion systems—such as Fluigent’s Omi platform—are designed to replicate tissue-specific environments by providing precise, programmable flow control that maintains barrier integrity, tissue architecture, and long-term viability. It supports repeated dosing and long-term cultures, enhancing predictive capability in drug screening assays. A pressure device from TissUse allows them to manipulated their in-house OoCs.
Learn more about capabilities of Omi, organ-on-a-chip fluidic platform.

Figure 6: Omi system under bright-field microscopy
Evolution microfluidic perfusion
Microfluidic perfusion is evolving from individual systems toward highly integrated, smart, and scalable platforms. Looking ahead, there are several trends that are emerging to transform the field:
- Complex Multi-Organ Integration & Closed-Loop Systems:
Expect a rise in “body-on-a-chip” ecosystems—networks of interconnected tissue chipsto model systemic physiological interactions. These may be coupled with tubeless manifold interfaces, to prevent leakage and bubble.
- Novel Materials & Additive Manufacturing:
Advances in smart polymers, and 3D printing will facilitate sophisticated custom geometries to allow bigger range of biomechanical simulations and flow control.
- Regulatory Momentum and Standardization:
As perfused micro physiological systems (e.g., organ-on-chip) gain regulatory acceptance, we’ll see standardized platforms entering drug development pipelines. The adoption of OoC in the US FDA Modernization Act 2.0 allows the useof the ogan-on-a-chip research to be able to translate to clinical applications.
Key Focus Areas for Continued Innovation:
- Bubble Formation & Flow Stability: Persistent issues with bubble nucleation remain; improved degassing techniques and real-time bubble detection are essential.
- Long-Term Sterility & System Robustness: Extended experiments demand closed systems with effective filtration, resilient seals, and designed-in redundancy to prevent microbial contamination
- Manufacturing Scale-Up & Cost: Moving from lab prototypes to commercial products necessitates durable, user-friendly systems that balance complexity with affordability—potentially through modular and reconfigurable units.
Future Vision: As these trends and solutions converge, microfluidic perfusion systems will evolve into intelligent, reliable, and accessible tools. Their adoption will extend beyond specialized labs into mainstream biomedical research, drug development, and personalized medicine—ushering in a new era of dynamic, physiologically relevant in vitro modeling.
Related Solutions
Related Resources
-
Microfluidics Case Studies Programmable Injections of Sweeteners with the Fluigent Flow-EZ for Tongue-on-a-chip Receptomics Taste Assay Read more
-
Microfluidic Application Notes Gut-on-Chip Model Development Using OOAC Platform, Omi Read more
-
Microfluidics Case Studies A multiplex microfluidic circuit for blood vessel-on-a-chip perfusion using Fluigent’s FlowEZ Read more
-
Microfluidics Case Studies Mimicking tumor microenvironment using a 3D microfluidic model to improve cancer investigations Read more
-
Microfluidics Case Studies Creating a Microfluidic Cancer-on-Chip Platform using Fluigent’s High Throughput Cell Perfusion Pack Read more
-
Microfluidic Application Notes Long-term fluid recirculation system for Organ-on-a-Chip applications Read more
-
Microfluidics Article Reviews Human Blood Brain Barrier (BBB) permeability -on-chip assessment Read more
-
Microfluidics White Papers A review of Organ on Chip Technology – A White Paper Read more
-
Expert Reviews: Basics of Microfluidics Why Control Shear Stress in Cell Biology? Read more
-
Microfluidic Application Notes Peristaltic Pump vs Pressure-Based Microfluidic Flow Control for Organ on Chip applications Read more
-
Expert Reviews: Basics of Microfluidics Mimicking in-vivo environments: biochemical and biomechanical stimulation Read more
-
Expert Reviews: Basics of Microfluidics Flow Control Technologies: Comparison between peristaltic, syringe and pressure pumps for microfluidic applications Read more
References:
- Horowitz LF, Rodriguez AD, Ray T, et al. Microfluidics for interrogating live intact tissues. Microsystems & Nanoengineering. 2020;6:69. doi:10.1038/s41378-020-0164-0 nature.com
- Hattori K, Sugiura S, Kanamori T. Pressure-driven microfluidic perfusion culture device for integrated dose-response assays. J Lab Autom. 2013 Dec;18(6):437–45. doi:10.1177/2211068213503155 pubmed.ncbi.nlm.nih.gov
- Sønstevold L, Koza P, Czerkies M, Andreassen E, McMahon P, Vereshchagina E, et al. Prototyping in polymethylpentene to enable oxygen-permeable on-a-chip cell culture and organ-on-a-chip devices suitable for microscopy. Micromachines. 2024;15(7):898. doi:10.3390/mi15070898 doi.org
- Nie J, Fu J, He Y. Hydrogels: The next generation body materials for microfluidic chips? Small. 2020 Nov;16(46):e2003797. doi:10.1002/smll.202003797 pubmed.ncbi.nlm.nih.gov
- Rho JY, Ashman RB, Turner CH. Young’s modulus of trabecular and cortical bone material: ultrasonic and microtensile measurements. J Biomech. 1993;26(2):111–19. doi:10.1016/0021-9290(93)90042-D researchgate.net
- Budday S, Nay R, de Rooij R, Steinmann P, Wyrobek T, Ovaert TC, et al. Mechanical properties of gray and white matter brain tissue by indentation. J Mech Behav Biomed Mater. 2015;46:318–30. doi:10.1016/j.jmbbm.2015.02.024 pubmed.ncbi.nlm.nih.gov
- Choi Y, Tran H-V, Lee TR. Self-assembled monolayer coatings on gold and silica surfaces for antifouling applications: a review. Coatings. 2022;12(10):1462. doi:10.3390/coatings12101462 mdpi.com
- Sun B, Xie K, Chen T-H, Lam RHW. Preferred cell alignment along concave microgrooves. RSC Adv. 2017;7:6788–94. doi:10.1039/c6ra26545f pubs.rsc.org
- Huber D, Oskooei A, Casadevall i Solvas X, de Mello AJ, Kaigala GV. Hydrodynamics in cell studies. Chem Rev. 2018;118(4):2042–79. doi:10.1021/acs.chemrev.7b00317 pubmed.ncbi.nlm.nih.gov
- Pereiro I, Fomitcheva Khartchenko AF, Petrini L, Kaigala GV. Nip the bubble in the bud: a guide to avoid gas nucleation in microfluidics. Lab Chip. 2019;19(14):2296–2314. doi:10.1039/c9lc00211a pubs.rsc.org
- Azimzadeh M, Khashayar P, Amereh M, Tasnim N, Hoorfar M, Akbari M. Microfluidic-based oxygen (O₂) sensors for on-chip monitoring of cell, tissue and organ metabolism. Biosensors. 2021 Dec 22;12(1):6. doi:10.3390/bios12010006 pmc.ncbi.nlm.nih.gov