Precision Microfluidics for Magnetic Nanoparticle Encapsulation
Encapsulating magnetic nanoparticles into droplets is increasingly being explored for its potential in biomedical and technological applications. This approach enables both precise droplet manipulation through magnetic fields and the encapsulation of magnetic nanoparticles for improved stability and functionality. Droplet-based microfluidics offers a precise and reproducible method to achieve this.
In this application note, we present the RayDrop™ (designed and developed by Secoya Tehcnologies), coupled with Fluigent’s Flow EZ controllers, to encapsulate iron oxide nanoparticles in monodisperse microcapsules.
What makes microfluidics ideal for the encapsulation of magnetic nanoparticles?
Droplet-based microfluidics has gained acceptance in medicine, agriculture, biosensing, catalysis, and environmental science. By producing monodisperse droplets in the nanoliter to picoliter range, it enables the formation of well-controlled microreactors with low sample consumption, high reproducibility and minimal risk of contamination. These characteristics make it particularly suitable for applications such as drug encapsulation, diagnostics, chemical synthesis, and cell culture. 1–4
The integration of droplets with magnetic nanoparticles (MNPs) offers a particularly promising approach, allowing precise control over droplet movement while simultaneously facilitating magnetic nanoparticle encapsulation within microfluidic environments. MNPs can be used to guide and control droplets remotely using magnetic fields, enabling operations like merging, splitting and sorting. Droplets can also encapsulate MNPs to ensure stability and reproducibility. Encapsulation prevents large aggregations, facilitates surface modification and improves targeting efficiency.1,3,5 This dual capability expands the range of possible applications in biomedical and chemical workflows.
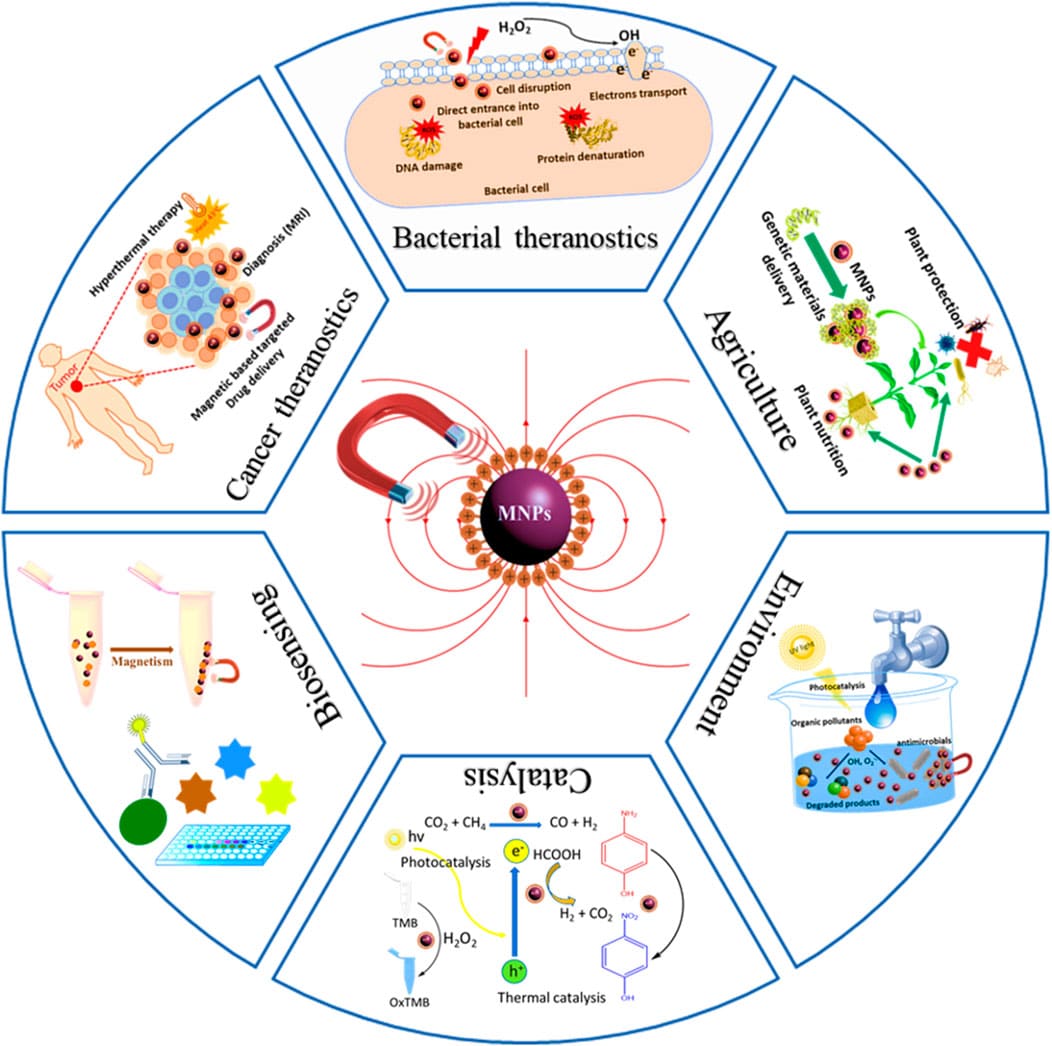
Figure 1. Diverse applications of magnetic nanoparticles4
Iron oxide nanoparticles are among the most widely studied MNPs due to their high magnetic susceptibility, biocompatibility, and chemical stability. Their size, comparable to proteins or viruses, allows precise control with external magnetic fields, while their surface properties enable functionalization with drugs, ligands or biomolecules. These features make them particularly relevant for targeted delivery, imaging, hyperthermia, and other biomedical applications. 1–3,6
To support these encapsulation strategies, Secoya Technologies and Fluigent have developed a dedicated solution combining the RayDrop droplet generator with Flow EZ pressure-based controllers. This system ensures stable and reproducible droplet formation, enabling reliable encapsulation of magnetic nanoparticles.
Reliable encapsulation of magnetic nanoparticles
Materials:
Magnetic nanoparticles coating:
- Iron oxide nanopowder Fe3O4, particle size 50-100 nm (SEM), 97% trace metals basis (637106, Sigma-Aldrich)
- 0.5M citric acid solution (Citric acid, 99%, C0759, Sigma Aldrich)
Reagents:
- Core phase:
- Distilled water
- Shell phase:
- 97% in wt. Poly(ethylene glycol) diacrylate average Mn 250 (PEGDA250, 475629, Sigma Aldrich) and 3% in wt. 2-Hydroxy-2-methylpropiophenone (Darocur, 97%, 405655, Sigma Aldrich).
- Continuous phase:
- 1% in wt. Poly(vinyl alcohol) Mw 9000-10000, 80% hydrolyzed (PVA, 360627, Sigma Aldrich) in DI water
Products:
- Microfluidic flow controller: Flow EZ
- Microfluidic flow sensor: Flow Unit
- Microfluidic double emulsion device: RayDrop Double Emulsion
- Microfluidic software control: The Link
- Real-time control and lab automation software: OxyGEN
Microfluidic setup:
The iron oxide nanoparticles are coated with citric acid to be suspended in an aqueous solution.
The setup for the generation of droplets is illustrated in the following Figure 2 using the UV-crosslinked microcapsule production platform. The Flow EZ are connected to the core phase, the shell phase and the continuous phase.
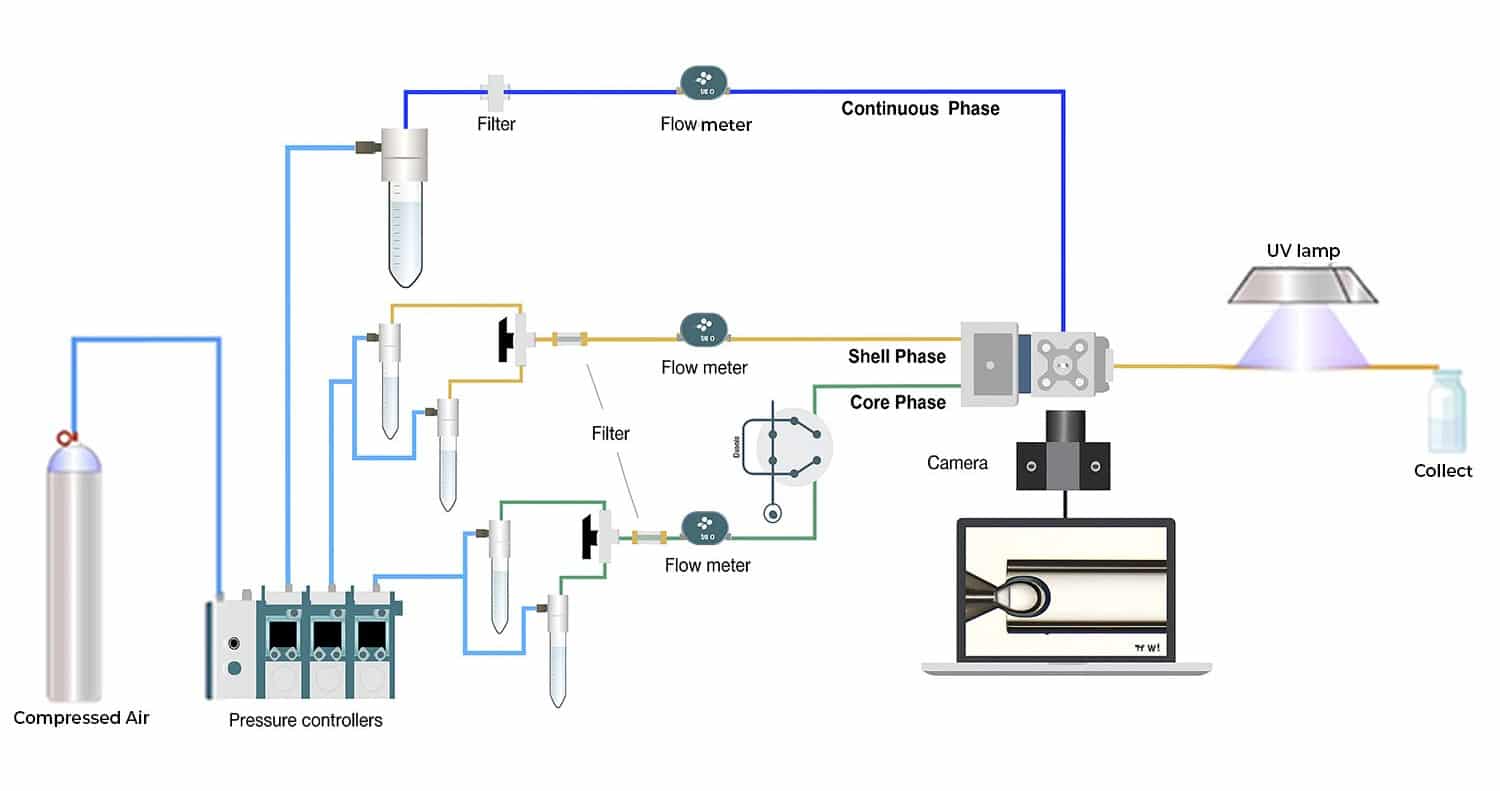
Figure 2. Scheme of the setup of encapsulated magnetic nanoparticles production
The microcapsules were produced using the RayDrop 90-160-450 µm (diameters of core, shell nozzles, and the collection capillary respectively).
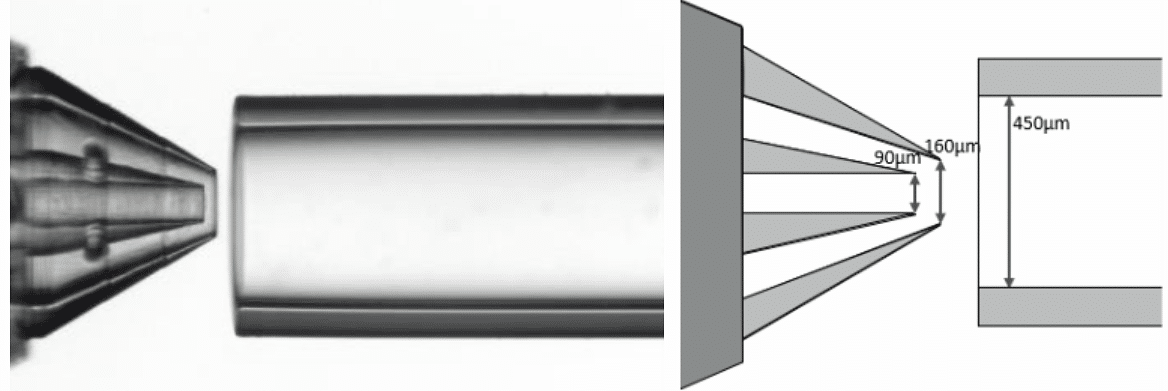
Figure 3. RayDrop picture with dimensions
Protocol: workflow for microfluidic encapsulation of magnetic nanoparticles
- All liquids are filtered to avoid clogging (pore size 0.2 µm).
- All solutions are de-gassed to minimize air bubbles inside the system.
Stage 0: Magnetic nanoparticle coating7
- Iron oxide nanoparticles are dispersed and coated with citric acid to improve stability.
Stage 1: Preparation
- The RayDrop is connected to Flow EZ controllers and purged before operation to ensure that all the channels leading to the system and to the waste outlet are wetted to purge air.
Stage 2: Single emulsion generation
- Shell material was first encapsulated alone in the continuous phase, increasing pressure until reaching jetting mode to validate droplet stability.
Stage 3: Double emulsion generation
- Production of a double emulsion of the shell phase by adjusting the core phase press, due to the shearing of this phase with the previous phase produced during single emulsion. Magnetic nanoparticles are encapsulated in the aqueous core phase.
Stage 4: Collection and cross-linking
- Once the droplets containing iron oxide nanoparticles are formed, UV irradiation triggers cross-linking, forming a stable 3D network.
- The UV module is positioned so droplets are visible at the outlet. Flow rates can be fine-tuned to ensure uniformity and stable production.
- The output of the UV module is collected into distilled water.
Magnetic Nanoparticles Encapsulation: Stability and Magnetic Performance Results
Encapsulation of magnetic nanoparticles was successfully achieved within the aqueous core of the microcapsules (Figure 3), across a concentration range of 10 to 40 g/L. The approximate size of microcapsules is 300 µm.
At 10 g/L (~1% m(MNPs)/m(capsule), the suspensions were stable and displayed moderate magnetic responsiveness. Increasing the concentration to 20 g/L (~2%) and 40 g/L (~4%) further improved stability, producing suspensions that were more responsive to an external magnet compared to sedimented samples. The ratio m(MNPs)/m(capsule) shows how much of the microcapsule’s total mass is made up of magnetic nanoparticles, indicating the level of nanoparticle loading.
Additional tests at 30 g/L confirmed sufficient responsiveness for attraction by a neodymium magnet. As shown in Figure 4, nanoparticles aligned along the magnetic field lines, and this alignment persisted after removal of the magnet, demonstrating strong interparticle interactions within the microcapsule core.
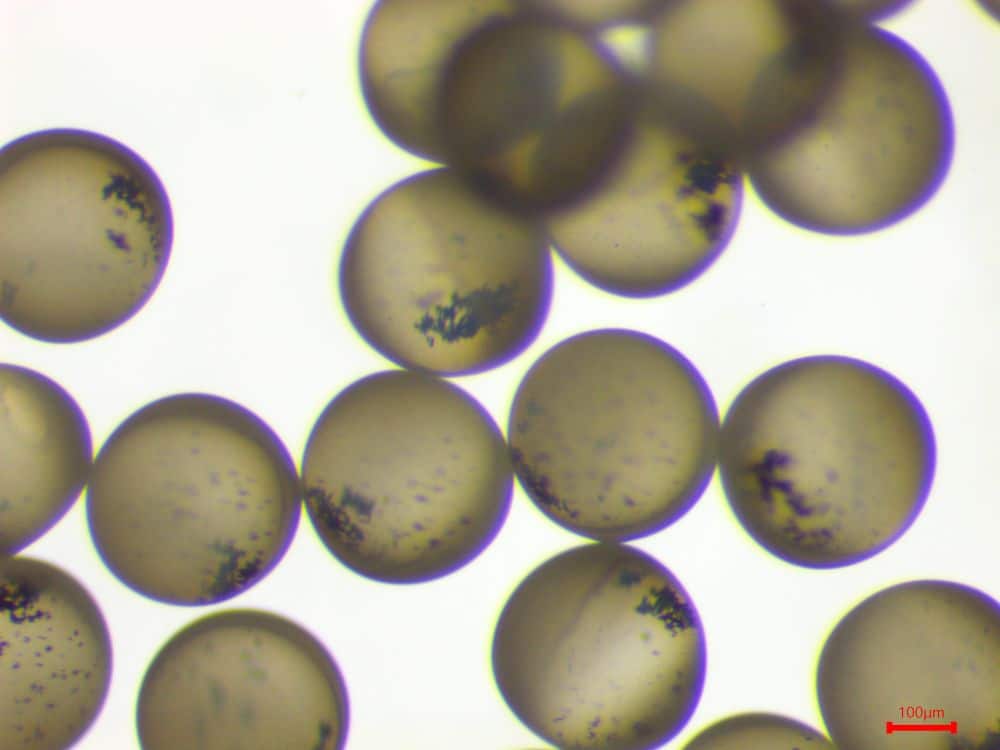
Figure 4. Encapsulation of iron oxide nanoparticles (before applying a magnetic field)
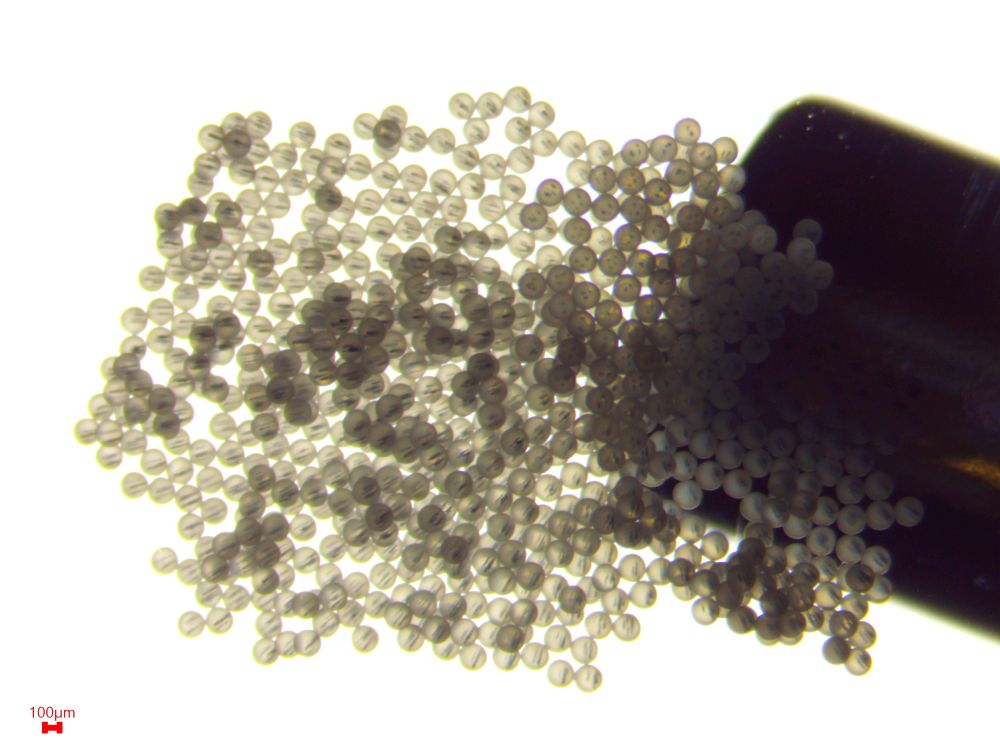
Figure 5. Alignment of encapsulated magnetic nanoparticles along magnetic field lines of a magnet
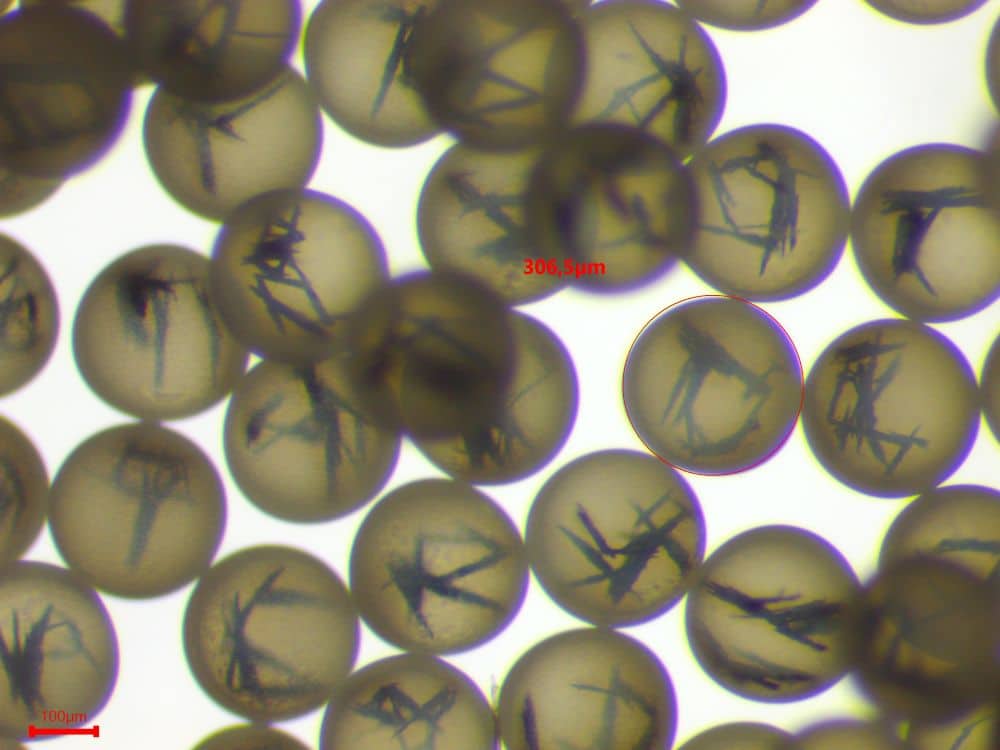
Figure 6. Encapsulation of iron oxide nanoparticles after exposure to a magnetic field
Conclusion
This work highlights the potential of droplet-based microfluidics for the encapsulation of magnetic nanoparticles into stable and magnetically active microcapsules. Using Secoya’s RayDrop and Fluigent’s fluid delivery technology, precise and reproducible droplet encapsulation can be achieved, with tunable nanoparticle concentrations. The magnetically responsive and stable suspensions produced are suitable for applications such as targeted drug delivery, biosensing and diagnostic platforms.
Ready to explore precise encapsulation for your magnetic nanoparticle research?
Contact our experts or explore Fluigent’s microfluidic systems to find the right solution for your application.
Related Products
Expertises & Resources
-
Expert Reviews: Basics of Microfluidics 10 Tips for Reliable Droplet Generation Read more
-
Microfluidic Application Notes Creating Microcapsules With PEGDA Hydrogel Read more
-
Microfluidics White Papers Double emulsion for the generation of microcapsules – a Review Read more
-
Microfluidic Application Notes Encapsulation of Cells In Small Double Emulsions Read more
-
Microfluidic Application Notes Agarose Microcapsules Synthesis Read more
-
Microfluidics White Papers Droplet-based Microfluidics – A Complete Guide Read more
-
Microfluidic Application Notes UV-Crosslinking of Microcapsules Read more
-
Expert Reviews: Basics of Microfluidics Flow control for droplet generation using syringe pumps and pressure-based flow controllers Read more
References
(1) Li, J.; Su, K.; Liu, H.; Zou, Y. Recent Advances in Magnetically Actuated Droplet Manipulation for Biomedical Applications. Magnetochemistry 2024, 10 (4), 28. https://doi.org/10.3390/magnetochemistry10040028.
(2) Lima-Tenório, M. K.; Gómez Pineda, E. A.; Ahmad, N. M.; Fessi, H.; Elaissari, A. Magnetic Nanoparticles: In Vivo Cancer Diagnosis and Therapy. Int. J. Pharm. 2015, 493 (1–2), 313–327. https://doi.org/10.1016/j.ijpharm.2015.07.059.
(3) Aisida, S. O.; Akpa, P. A.; Ahmad, I.; Zhao, T.; Maaza, M.; Ezema, F. I. Bio-Inspired Encapsulation and Functionalization of Iron Oxide Nanoparticles for Biomedical Applications. Eur. Polym. J. 2020, 122, 109371. https://doi.org/10.1016/j.eurpolymj.2019.109371.
(4) Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. https://doi.org/10.3389/fchem.2021.629054.
(5) Madhulatha, A. V. S.; Susithra, E.; Gunda, R. K. Development of Magnetic Nanoparticles and Encapsulation Methods – An Overview. Indian J. Pharm. Educ. Res. 2022, 56 (1), 01–08. https://doi.org/10.5530/ijper.56.1.1.
(6) Cheraghipour, E.; Javadpour, S.; Mehdizadeh, A. R. Citrate Capped Superparamagnetic Iron Oxide Nanoparticles Used for Hyperthermia Therapy. J. Biomed. Sci. Eng. 2012, 05 (12), 715–719. https://doi.org/10.4236/jbise.2012.512089.
(7) Khan, S.; Shah, Z. H.; Riaz, S.; Ahmad, N.; Islam, S.; Raza, M. A.; Naseem, S. Antimicrobial Activity of Citric Acid Functionalized Iron Oxide Nanoparticles –Superparamagnetic Effect. Ceram. Int. 2020, 46 (8), 10942–10951. https://doi.org/10.1016/j.ceramint.2020.01.109.
Written by Maï Loan LE in collaboration with Secoya Technologies