Alginate Microcapsule Synthesis
Microencapsulation is a method to encapsulate different functional materials at nano- and micro-scales, which can provide the necessary protection for the encapsulated materials (1).
Alginate microcapsule synthesis is currently expanding due to the extensive use of alginate, which is widely used as natural polymers in pharmaceutical, food and cosmetic industries. Alginate microcapsules are biocompatible, biodegradable and nontoxic carriers applied to encapsulate active substances, release drugs in a controlled way, enhance bioavailability, deliver supplements, and more(2).
The following is a method for the encapsulation of reagents into alginate microcapsules with total control of droplet size and core-shell ratio accomplished by the RayDrop (developed and manufactured by Secoya) and Fluigent pumping technology.
Secoya developed and manufactured the RayDrop used to perform this application note.

Introduction to alginate microcapsule
Recent advances in life science technology have prompted the need to develop microcapsule delivery systems that can encapsulate many different functional or active materials.. The application of biodegradable microcapsule systems can not only effectively prevent the degradation of core materials in the body or the biological environment, but also improve the bioavailability, control release, and prolong the half‐time or storage of core active materials (3).
In this application note, a microfluidic approach is developed to fabricate monodisperse alginate microcapsules with oil cores, which have the potential to be a brand-new type of vehicle for encapsulating, storing and/or transferring lipophilic drugs or active ingredients/chemicals.
In alginate microcapsule synthesis, the microcapsules with oil cores are generated in the RayDrop, a microcapillary microfluidic device based on the use of a 3D-printed injection nozzle carrying two fluids, the core and the shell phases. This is positioned in front of an extraction capillary in a cavity filled with the third (continuous) phase. Fine control of the fluid flows leads to defined capsule and shell dimensions.
Both the alginate microcapsule size and the thickness of alginate membrane can be easily controlled by modulating the dimensions of the microfluidic device and the flow rate of the solutions because the outer diameter of the O/W/O double emulsion templates and the size of their inner oil cores can be controlled independently by adjusting the inner diameters of emulsification tubes and the flow rates of different solutions (4,5).
Highly monodispersed alginate microcapsules are produced by gelling O/W/O emulsions in oil solution with acetic acid, where the pH decreasing will trigger the calcium ions being released from calcium complex and cross-linking with alginate molecules (4).
Materials and methods to produce alginate microcapsules
MATERIALS
Reagents
– Core phase: MCT1 (MCT oil)
– Shell phase:
Al1 MiliQ water with 1% w/w TWEEN 80 and 1%w/w Alginate and 2:1 molar ratio of CaEDTA
Al2 MiliQ water with 1% w/w TWEEN 80 and 2%w/w Alginate and 2:1 molar ratio of CaEDTA
Al3 MiliQ water with 1% w/w TWEEN 80 and 3%w/w Alginate and 2:1 molar ratio of CaEDTA
– Continuous phase: MCT3 (MCT with 2% w/w PGPR (E576) and 5% acetic acid).
Products/Instruments
METHODS: Working principle of microfluidic alginate microcapsule synthesis
EXPERIMENT SEQUENCE
Stage 0: Preparation
» Filter all liquids to avoid clogging (pore size 0.2 µm).
» De-gas solutions to minimize the apparition of air bubbles inside the system.
Stage 1: Shell Phase Simple Emulsion
» Production of a single emulsion of the shell phase in the continuous phase, increasing the pressure until reaching a jetting mode.
Stage 2: Double Emulsion
» Production of a double emulsion adjusting the core flow, due to the shearing of this phase with the previous phase (single emulsion droplet already formed).
Stage 3: Droplet collection and microcapsule precipitation (crosslinking).
» Once the droplets were formed, the cross-linking procedure was performed.
» The collected material is allowed to reside in MCT 3 solution. This enables complete crosslinking of the alginate shell.
» After cross-linking is complete, the microbeads are collected on a filter/sieve, washed with excess water and resuspended in a MCT 1 working solution.
Stage 4: Production run
Oil core – hydrogel shell microcapsules are produced at gram quantities. The system was left running for at least 30 minutes to determine the long-term stability and ability to withstand clogging.
Results
Al1 and Al2 resulted in the successful formation of oil core–alginate shell microbeads. The core to droplet ratio has altered, resulting in larger cores. This could be due to the interaction of alginate with the oil phase or the retardation of shell formation owing to the viscosity of alginate.
As can be seen in Table 1, Al3 (3% alginate solutions) did not result in stable droplet formation as the concentration was too viscous.
For a configuration with the nozzle and output capillaries (respectively 90µm, 160µm and 450µm) as presented in this note, adjusting the flow rates of the fluids allows for fine control of the capsule dimensions. With this setup, microcapsules from 212µm- 245µm can be easily produced (see Figure 4 and Figure 5). The shell thickness of microcapsules can vary by changing the ratio of the flow rates of the shell and core phases. Here, core thickness varies from 75µm to 173µm.
| Working solution | Core flow rate µL/min | Shell flow rate µL/min | Continuous flow rate µL/min | Droplet size /µm | Core size/µm | Droplet formation rate /Hz |
| Al1 | 20 | 60 | 100 | 221 | 75 | 236 |
| Al1 | 30 | 80 | 150 | 271 | 124 | 176 |
| Al1 | 50 | 20 | 100 | 222 | 152 | 204 |
| Al1 | 50 | 40 | 150 | 234 | 127 | 273 |
| Al2 | 20 | 60 | 100 | 215 | 77 | 256 |
| Al2 | 30 | 80 | 150 | 245 | 85 | 238 |
| Al2 | 50 | 20 | 100 | 212 | 141 | 234 |
| Al2 | 50 | 40 | 150 | 239 | 173 | 210 |
| Al3 | – | – | – | – | – | No Droplets |
| Al3 | – | – | – | – | – | No Droplets |
| Al3 | – | – | – | – | – | No Droplets |
| Al3 | – | – | – | – | – | No Droplets |
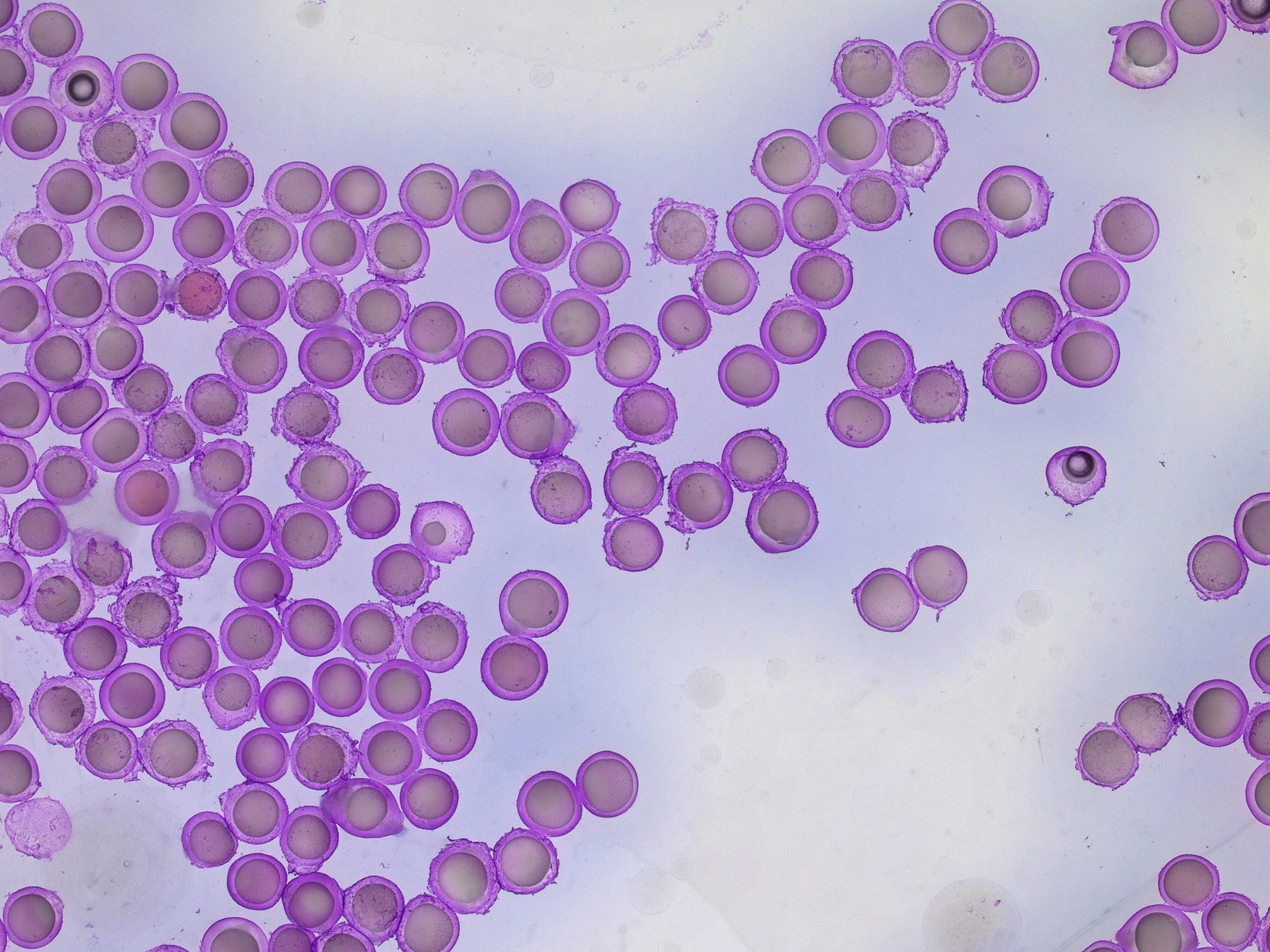
Conclusion
Microfluidic technology adaptation for alginate microcapsule production has seen significant growth in varied application fields. The benefits of reproducibility, real-time control and reduction of waste are leading users to switch from conventional batch methods to microfluidics.
In this application note, made in collaboration with Small Biotechnologies, the complex emulsion production platform generated highly monodispersed alginate microcapsules with a size between 212 and 245 µm with standard RayDrop™ configuration (Nozzle of 90µm and outlet capillary 150µm) using an alginate solution of 1% in water. Other concentrations of alginate such as 2%, which is widely used in the delivery of small chemical drugs, protein delivery, and wound dressings, have been successfully tested by following the same process.
In this alginate microcapsule synthesis application, we have demonstrated that microcapsule size variation can be successfully obtained by altering the flow rates of the phases and/or the size of the Raydrop™ chips. We have also demonstrated that core-shell ratio can be successfully adjusted by altering the relative flow rates of core and shell phases.
Related Product
Related Resources
-
Expert Reviews: Basics of Microfluidics Microfluidics in Drug Delivery: A New Era of Precision Medicine Read more
-
Expert Reviews: Basics of Microfluidics 10 Tips for Reliable Droplet Generation Read more
-
Microfluidics White Papers Double emulsion for the generation of microcapsules – a Review Read more
-
Microfluidics White Papers Droplet-based Microfluidics – A Complete Guide Read more
-
Microfluidic Application Notes Alginate Microbeads Production Read more
REFERENCES
- Bah, M.G., Bilal, H.M. and Wang, J. (2020) “Fabrication and application of complex microcapsules: A Review,” Soft Matter, 16(3), pp. 570–590. Available at: https://doi.org/10.1039/c9sm01634a.
- Paques, J.P. et al. (2014) “Preparation methods of alginate nanoparticles,” Advances in Colloid and Interface Science, 209, pp. 163–171. Available at: https://doi.org/10.1016/j.cis.2014.03.009.
- Chong, D., Liu, X., Ma, H., Huang, G., Han, Y., & Cui, X. et al. (2015). Advances in fabricating double-emulsion droplets and their biomedical applications. Microfluidics And Nanofluidics, 19(5), 1071-1090. doi: 10.1007/s10404-015-1635-8 11.
- Mørch, Ý.A. et al. (2006) “Effect of ca2+, ba2+, and sr2+ on alginate microbeads,” Biomacromolecules, 7(5), pp. 1471–1480. Available at: https://doi.org/10.1021/bm060010d.
- Zimmermann, H., Shirley, S.G. and Zimmermann, U. (2007) “Alginate-based encapsulation of cells: Past, present, and future,” Current Diabetes Reports, 7(4), pp. 314–320. Available at: https://doi.org/10.1007/s11892-007-0051-1.