5 Key Tips for Starting Organ-on-Chip Models
Transitioning from in vitro or animal models to Organ-on-Chip (OoC) platforms requires adapting to new paradigms of experimental design and interpretation. OoC systems introduce dynamic cellular and biochemical interactions that could differ significantly from traditional models. To succeed in implementing Organ-on-a-chip technology, it is important to understand how to leverage this unique strength to generate meaningful results.
Tip 1: Reframe Your Experimental Question for the Organ-On-Chip Model Context
In murine models, the systemic environment including immune responses and metabolism are within the model. In OoC models they are deliberately recreated. When shifting to microphysiological models, it’s essential to grasp how fluid flow, shear stress, and cell–cell/tissue–tissue interactions shape biological outcomes.
Aligning the research question with these parameters ensures that the model best utilizes the technology’s capabilities and generates physiologically relevant data.
Parameters to define your biological aim:
- Are you modeling barrier function, inflammation, drug metabolism, or intercellular signaling?
- Do you aim to replicate a single organ or multi-organ physiological interactions?
- Will you use primary cells, immortalized cell lines, or patient-derived iPSCs?
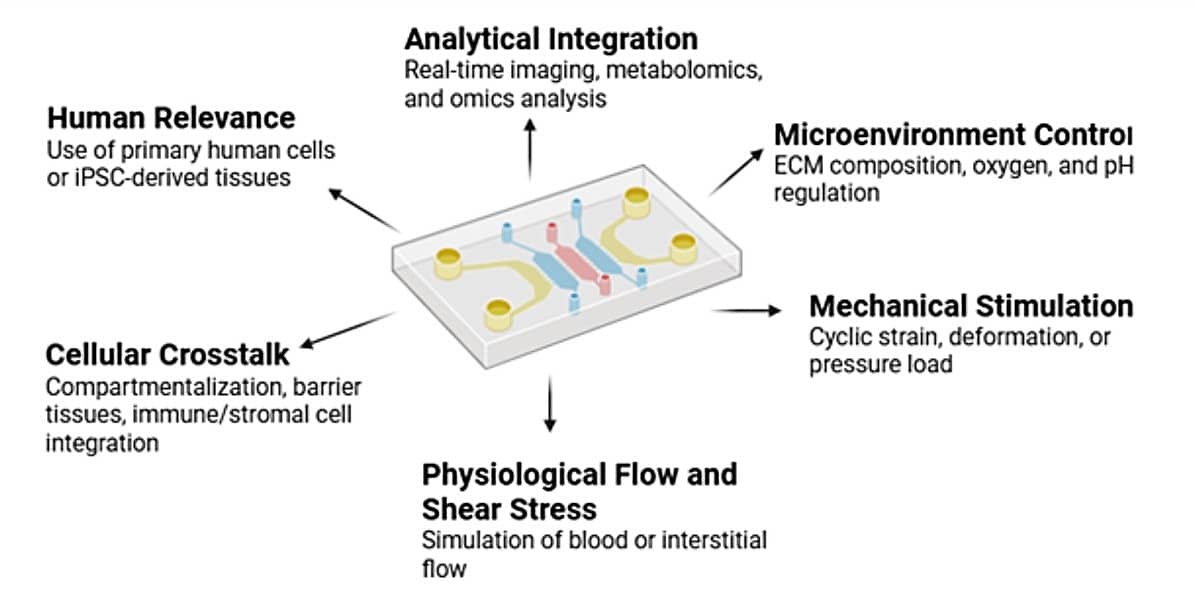
Figure 1. Organ-on-chip Models Design Parameters to Consider
Tip 2: Avoid Over-Engineering: Balance Complexity and Biological Relevance
Early-stage applications often benefit from a simpler setup. Overly complex designs, for example with multiple organs, hydrogel compartments, and branched perfusion, could obscure key biological questions and overcomplicate interpretation. We advise to ‘Start simple’. Single-organ chips tend to be easier to fabricate and maintain, while multi-organ systems (body-on-a-chip”) demand precise inter-organ flow control and shared media composition.
With a dual-organ system, drug toxicity can be studied., A combined liver-heart model can uncover off-target drug effects but requires controlling flow, perfusion and nutrient delivery [1].
- Design pragmatically
Tailor your model to your available lab equipment and infrastructure (incubators, microscopes, temperature controller).
- Leverage exiting technology
Recent advances allow researchers to use commercially available chips, reducing dependence on PDMS-based soft lithography and difficulties with chip assembly.
Note: Some applications benefit from specific materials and fabrication methods
- Glass chips: ideal for hypoxia or chemical resistance studies.
- PDMS chips: useful for oxygen permeability and mechanical deformation.
Read further: How to choose a microfluidic chip?
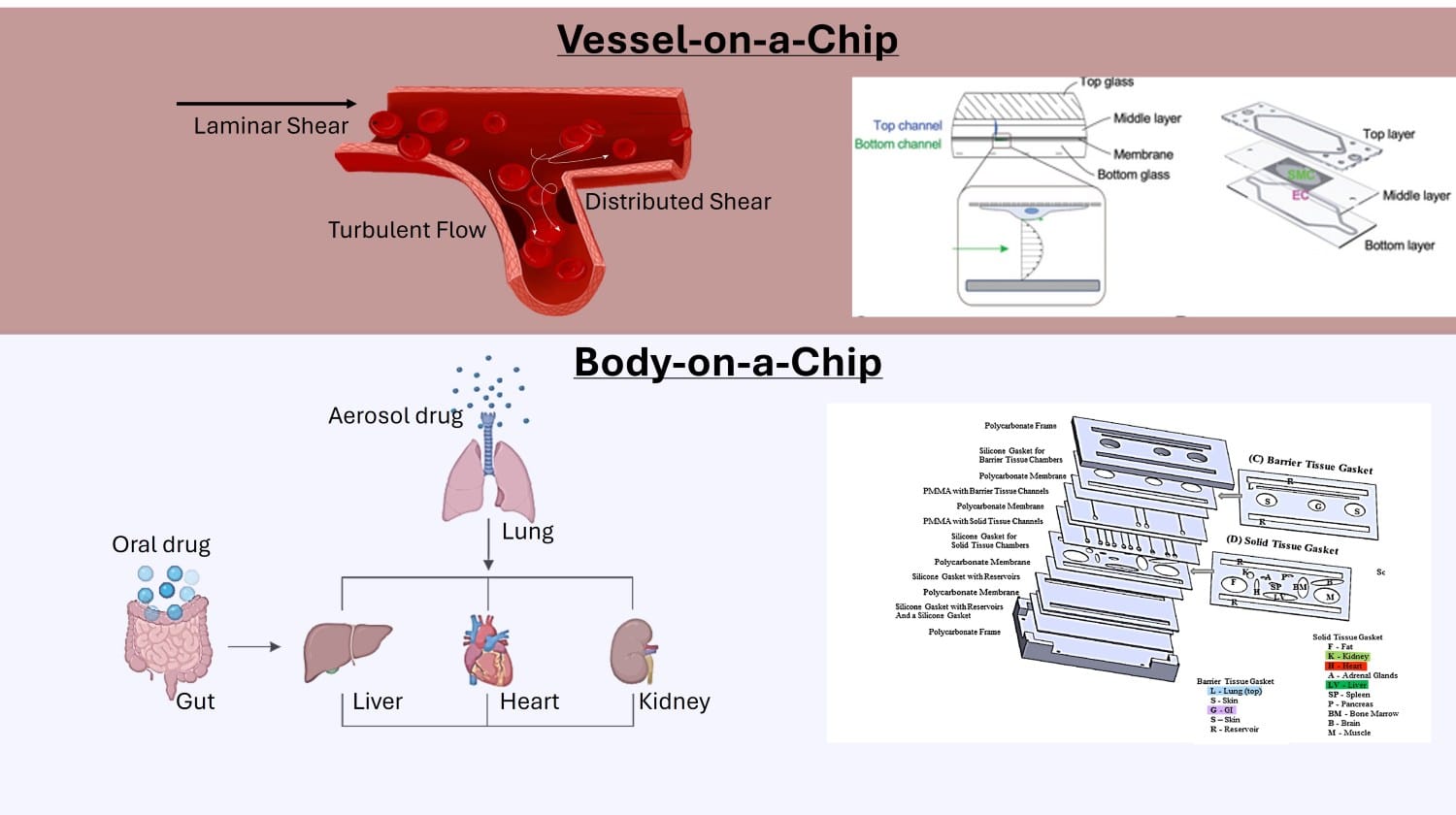
Figure 2. Representation of Single-organ system (Vessel-on-a-Chip) and Multi-organ system (Body-on-a-Chip) [3] [4]
The long-term vision of Organ-on-a-Chip Models is to develop an integrated ‘human body on a chip’ that replicates inter-organ communication and whole-body physiology. Current research shows that single and dual organ models form foundations for this goal[2]. Starting with barrier models such as the or brain-blood barrier or vessel-on-a-chip model, enables precise control of the microenvironment and robust validation of physiological relevance. Focused platforms are a first step towards scalable, multi-organ systems that could replace animal models in disease research and toxicity testing.
Tip 3: Align design to physiology
The development of an Organ-on-chip model begins with identifying the physiological process to be studied. Tissues are generally categorized into four main types, based on cellular organization, function and ECM characterization:
- Epithelial Tissue: cell sheets for protection and barrier function
- Connective Tissue: rich in ECM, support function
- Muscle Tissue: contractile fiber for generating movement and pressure
- Nervous Tissue: excitable neurons for signal transmission
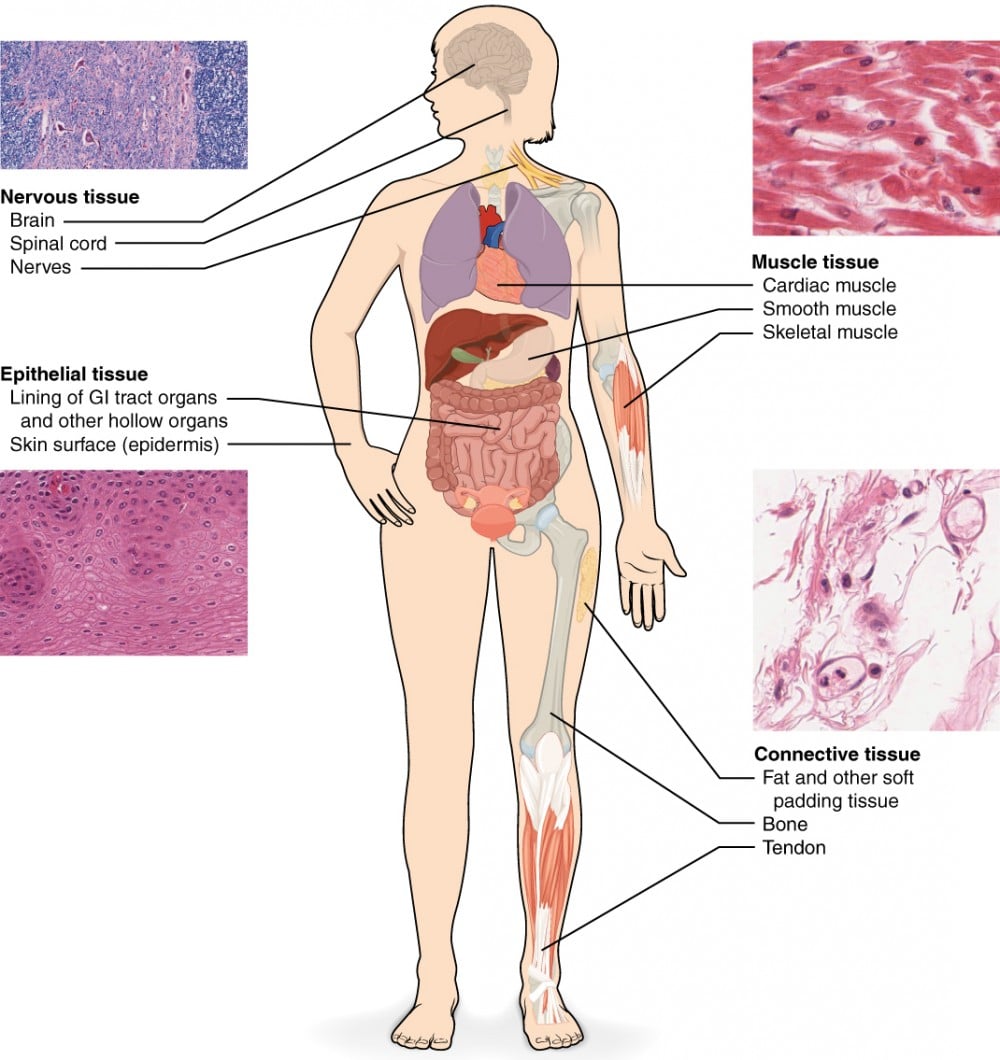
Figure 3. The four types of tissues are exemplified in nervous tissue, stratified squamous epithelial tissue, cardiac muscle tissue, and connective tissue in small intestine from [5]
It is important to integrate organ-specific microenvironmental cues, for example endothelial barriers for vasculature and organ-relevant ECM scaffolds.
1. Key Organ Functions and Microenvironments:
Design your chip around the core physiological processes you want to study depending on the organs and mechanisms involved. Examples of integrated physical cues that drive physiological functions:
- Vascular models: Apply controlled laminar flow and shear stress (1–10 dyn/cm²) across endothelial barriers to promote alignment and tight junction formation.
- Kidney-on-chip: Use perfused tubular channels with epithelial–endothelial co-cultures and ECM scaffolds to mimic reabsorption and selective permeability.
- 3D hydrogels or organoid cultures are used to promote natural morphology, function, and cell-cell interactions
Ensuring that physical and biochemical cues are consistent with the target organ physiology can increase the predictive relevance of the model.
Tip 4: Select Robust Fluidic Control Systems
Nearly all Organ-on-Chip designs include some media perfusion for media refreshment and support of the dynamic environment. Cell culture protocols similar to conventional in vitro systems can be adapted, but the addition of controlled flow enables nutrient renewal and mechanical stimulation. Using compartment-specific media and low-serum or defined formulations often reduces experimental variability.
Here links of the relevant product:
Flow control technologies:
Pressure-driven flow control is particularly relevant for Organ-on-Chip systems, as it enables stable, pulse-free perfusion that closely replicates physiological fluid dynamics. These systems achieve rapid stabilization (<1 ms) and high reproducibility (<0.1% CV), ensuring that cells experience consistent shear stress and nutrient delivery.
In contrast, syringe pumps generate pulsatile flow (~0.35% CV) and slower response times, which can introduce mechanical artifacts and variability in cell responses. By integrating feedback-controlled pressure regulation, researchers can finely tune flow profiles, oscillations, and temporal gradients, allowing precise simulation of biological rhythms such as vascular pulsation or periodic drug exposure—key factors in achieving physiologically relevant organ-on-chip models.
Read further on the pressure-based systems in organ-on-chip research.
Tip 5: Employ Continuous Analytics _ From Organ-on-a-chip to Lab-on-chip
Embedding analytical capabilities within Ooac systems enhances data quality and interpretability. Sensors for oxygen, pH, and transepithelial electrical resistance (TEER) can be incorporated to monitor the microenvironment in real time. Perfusate samples can be analyzed using ELISA, HPLC, or mass spectrometry to quantify metabolites or cytokines or other effects of drug exposure. PDMS and glass chips generally support optical transparency, enabling live-cell and confocal imaging.
Routine calibration, bubble prevention, and stable flow maintenance contribute to system reliability. Long-term control and measurement of flow rate, pressure, and cell viability is often necessary to ensure reproducibility.
Summary Table
| Aspect | Traditional Model | Organ-on-Chip Equivalent | Adjustment Needed |
|---|---|---|---|
| Biological Context | Whole organism (mice) | Isolated functional unit | Focus on microphysiology |
| Environment Control | Self-regulated | Engineered (flow, shear, O₂) | Develop microfluidic control |
| Complexity | Intrinsic | Designed | Begin simple, expand as needed |
| Cell Source | Mouse / 2D lines | Human / iPSC / 3D | Increase physiological fidelity |
| Readouts | Endpoint (blood/tissue) | Continuous, real-time | Integrate sensing and sampling |
| Validation | In vivo physiology | Microphysiological mimicry | Cross-validate across systems |
Start with a small, well-defined physiological question that bridges your existing mouse data and new organ-on-chip models. This approach will maximize interpretability and accelerate your understanding of how OoCs can complement, not just replace, your current research pipeline.
Related content
-
Microfluidic Application Notes Liver–Kidney Organ-On-Chip Model using the Omi™ Dual Platform Read more
-
Expert Reviews: Basics of Microfluidics The Role of Microfluidics in Advanced Organoid Modeling: from Static to Dynamic Read more
-
Microfluidics Case Studies Gut-on-Chip Modeling: From Chip Development to Perfusion Read more
-
Expert Reviews: Basics of Microfluidics Optimizing Microfluidic Perfusion: Best Practices and Innovations Read more
-
Microfluidics Case Studies Creating a Microfluidic Cancer-on-Chip Platform using Fluigent’s High Throughput Cell Perfusion Pack Read more
-
Expert Reviews: Basics of Microfluidics Pressure-Controlled Microfluidics in Organ-On-A-Chip Research Read more
-
Microfluidic Application Notes Long-term fluid recirculation system for Organ-on-a-Chip applications Read more
-
Microfluidics White Papers A review of Organ on Chip Technology – A White Paper Read more
-
Expert Reviews: Basics of Microfluidics Why Control Shear Stress in Cell Biology? Read more
References
1. Ferrari E, Visone R, Monti E, Torretta E, Moretti M, Occhetta P, et al. LivHeart: A Multi Organ-on-Chip Platform to Study Off-Target Cardiotoxicity of Drugs Upon Liver Metabolism. Advanced Materials Technologies. 2023;8(8):2201435.
2. Ingber DE. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat Rev Genet. 2022 Aug;23(8):467–91.
3. Paloschi V, Pauli J, Winski G, Wu Z, Li Z, Botti L, et al. Utilization of an Artery‐on‐a‐Chip to Unravel Novel Regulators and Therapeutic Targets in Vascular Diseases. Adv Healthcare Materials. 2024 Mar;13(6):2302907.
4. Miller PG, Shuler ML. Design and demonstration of a pumpless 14 compartment microphysiological system. Biotechnol Bioeng. 2016 Oct;113(10):2213–27.
5. Kabelik D. Unit 6: Tissue Structure and Functions. 2024 Aug 30 [cited 2025 Oct 17]; Available from: https://human-anatomy-i.pressbooks.tru.ca/chapter/unit-6-tissue-structure-and-functions/