Assess Cell Proliferation Using Pressure as a Tool
This application presents a simple method to use pressure as a tool to evaluate and monitor cell proliferation in microfluidic chips in real time. This is demonstrated experimentally using a custom microfluidic chip, on which it has been possible to observe and determine, under identical conditions, the correlation between the applied pressure and the number of cells.
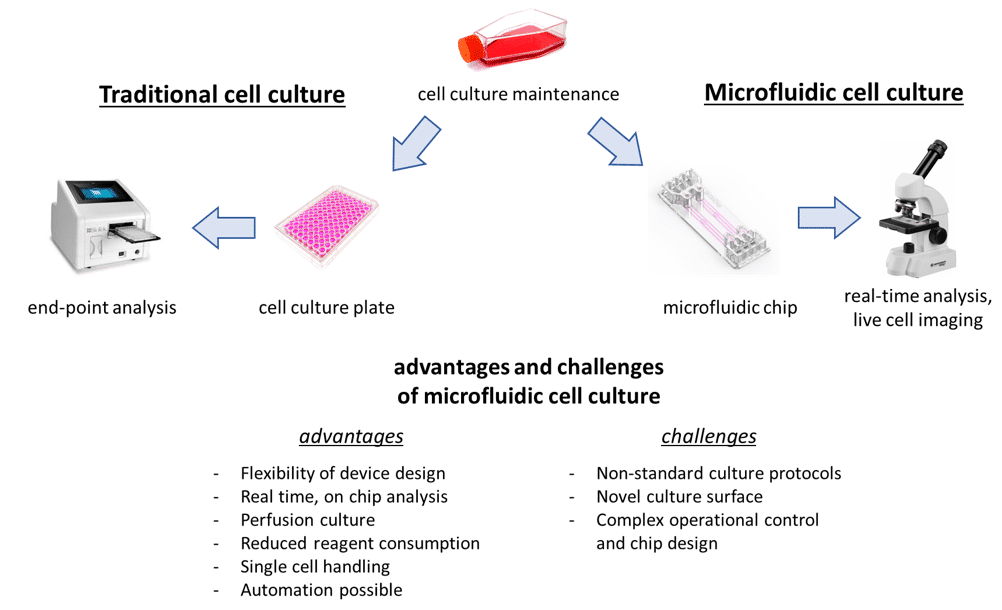
Cell morphology was studied under flowing and static culture condition in order to evaluate the influence of flow rate on cells and actin network development under these different conditions, which will lead to changes in cell heterogeneity, and thus in the way cells differentiate and proliferate.
Cell growth can be used to assess normal cell health, to measure responses to toxic insult, or as a prognostic and diagnostic tool in several cancers.
This study has been made in collaboration with Taha Messlmani, co-supervised by Fluigent and Anne Le Goff, from the Biomecanic and Bioengineer laboratory (BMBI – UMR CNRS 7338) of Université de Technologie de Compiègne (UTC).

Enhancing Cell Proliferation Monitoring with Pressure Controllers
Why Measure Cell Proliferation?
Cell proliferation serves as a crucial indicator of cell health and viability. Monitoring the proliferation rate is essential for assessing whether cells are actively dividing and growing as expected. Variations in proliferation rates may signify potential issues such as cell death, cellular stress, or the presence of toxins.
Additionally, insights into the kinetics of cell division, factors influencing cell growth, and the regulation of cellular processes are essential for understanding normal development, tissue regeneration, and disease progression.
Common Methods for Evaluating Cell Growth
Several widely used methods exist for evaluating cell proliferation:
- Cell Counting:
Manual counting using a hemocytometer or automated cell counters is a basic yet effective method. However, it can be time-consuming and subjective.
- DNA Synthesis Detection:
Since DNA replication is a fundamental event in cell multiplication, techniques that measure DNA synthesis can indirectly assess cell proliferation.
- Flow Cytometry:
Flow cytometry is a versatile technique involving labeling cells with fluorescent dyes like carboxyfluorescein succinimidyl ester (CFSE), which dilute as cells divide. Flow cytometry can measure DNA content using DNA-binding dyes (e.g., propidium iodide) to determine cell cycle phases.
The choice of method depends on factors such as cell type, research goals, and available resources. Researchers often combine these techniques for a comprehensive understanding of cell proliferation.
Innovative Approach:
We propose an alternative method for real-time cell growth monitoring, coupled with precise flow rate control within a microfluidic chip. This method relies on calculating hydrodynamic resistance and necessitates the use of pressure controllers alongside flow sensors to regulate the flow rate. This novel approach enhances the accuracy and efficiency of cell proliferation assessments in diverse research contexts.
Best Method for Measuring Cell Proliferation
The method determines cell proliferation by measuring the pressure increase using a pressurebased microfluidic system coupled to a flow sensor and using the equation ∆P= R x Q. Discover the complete protocol on the application note.
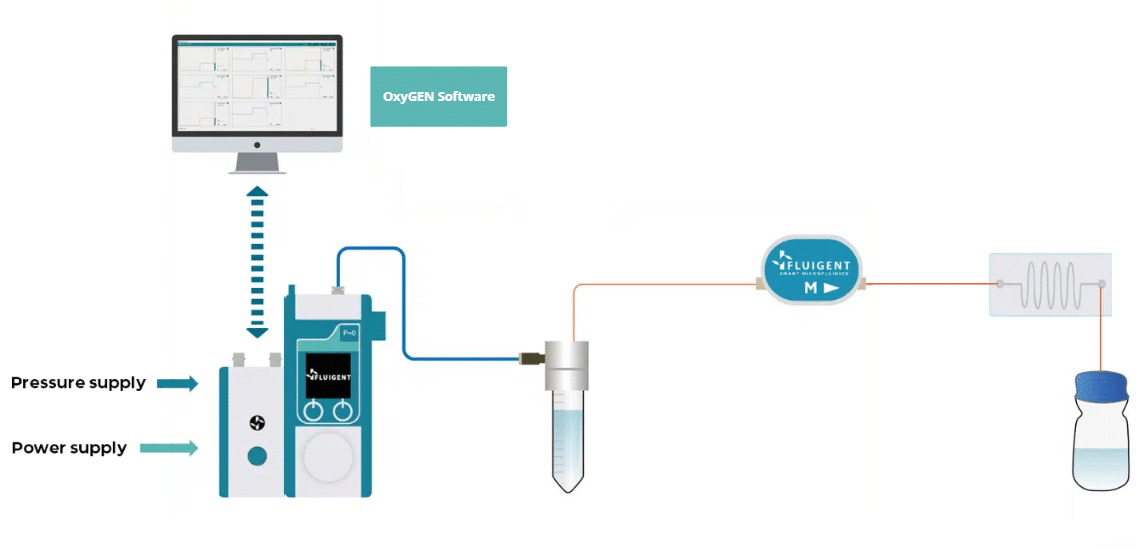
- Flow-EZ: The Flow EZ is the most advanced flow controller for pressure-based fluid control. It can be combined with a Flow Unit to control pressure or flow rate. A range of 10 – 40 mbar was used during the experiments.
- Flow unit M: A flow sensor that allows real time flow rate measurement up to 80 µL/min. By combining a Flow Unit with the Flow EZ, it is possible to switch from pressure control to flow rate control.
- A microfluidic cell culture chamber chip. At least 2 chips should be used for the first calibration.
- Tubing
- Cell culture media
- Cell line
Partial results
Determining cell proliferation within the biochip in real-time
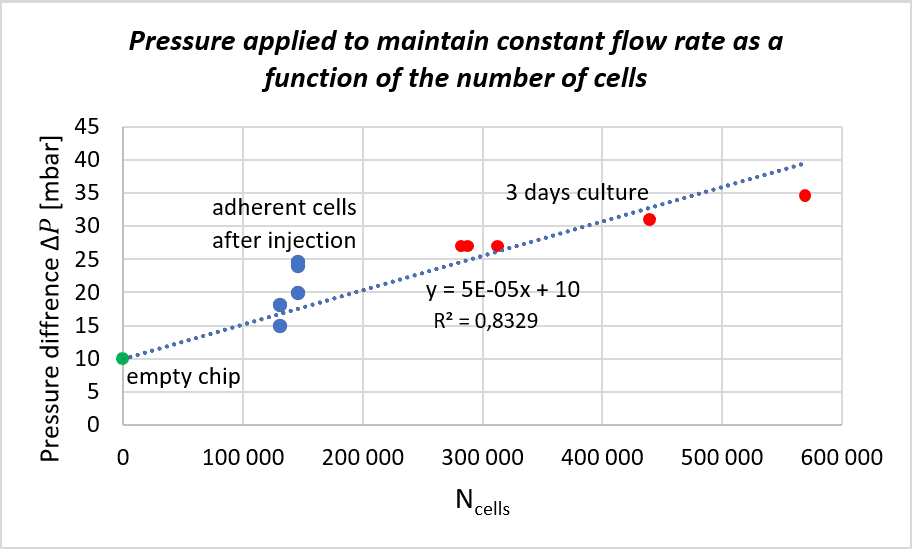
We followed the protocol to find the correlation between cell proliferation and the pressure increase for maintaining a steady flow rate. The experiment was repeated on 5 biochips to increase statistical significance. The pressure applied to maintain a flow rate of 10 µL/min as a function of the number of cells (estimated after injection and counted after 3 days of perfusion) is shown in the figure.
We can observe a correlation between the pressure applied and cell number for cells cultured for 3 days and counted afterwards. The slope from the curve was determined and lead to a linear function making it possible to estimate the number of cells, and therefore, cell growth, as a function of the applied pressure under identical conditions.
Cell viability under steady and dynamic flow conditions
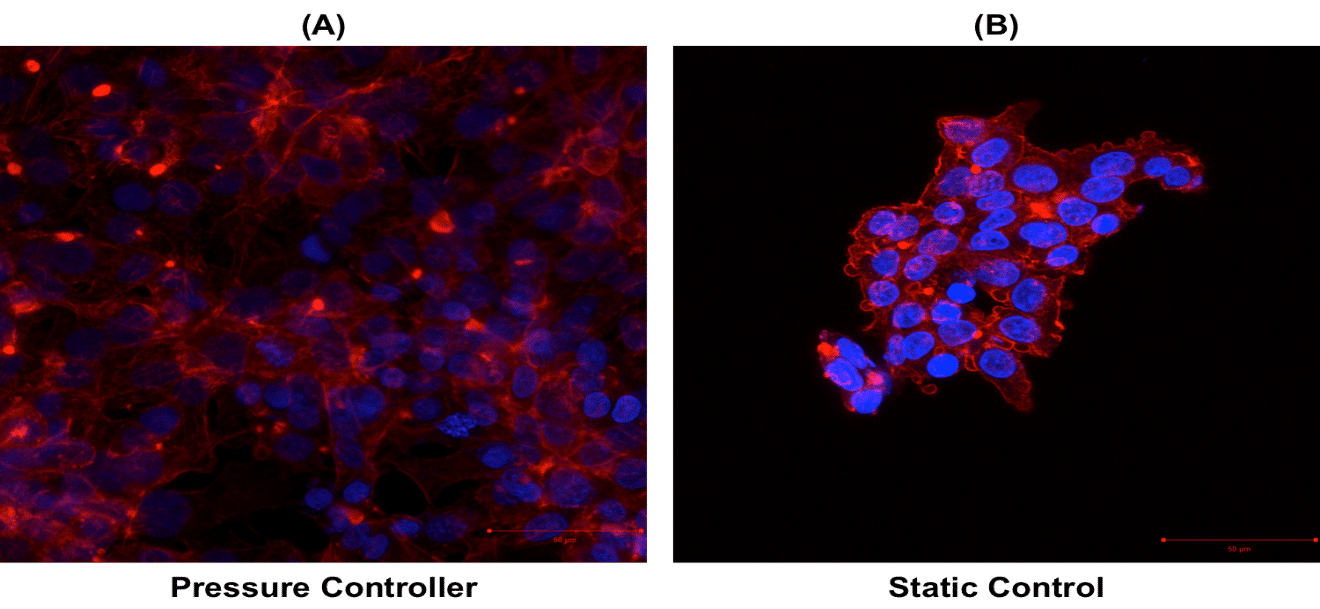
To assess the influence of flow rate on cells, cell morphology of cells cultured under dynamic and static conditions were compared (images on the right).
We observe that the actin network is more developed under dynamic conditions compared to static conditions. In fact, under flowing (dynamic) conditions, a low shear stress is applied on cells.
This shear stress tends to elongate cells, and as consequence, 2-dimensional cell proliferation is favored. Under static conditions, 3-dimensional cell growth is favored as no shear is applied. Cells growing 3-dimensionally could lead to increased cellular heterogeneity as they do not have access to the same amount of nutrients or oxygen within the microfluidic chamber. Under dynamic conditions, cells are in a favorable growth environment that is a continuous and homogeneous perfusion culture with steady and low shear stress.
Know more on Why is it important to control shear stress in your microfluidic experiments?
Conclusion
We demonstrated the use of pressure controllers coupled with flow sensors for determining and estimating cell proliferation within a microfluidic chip in real-time. The user can track, in real-time, cell proliferation by simply monitoring pressure increase. This method allows one to estimate cell proliferation kinetics within a chip in an inexpensive fashion. This system shows great advantages as it offers real time information on pressure and flow rate without requiring the preparation of additional replicates dedicated to monitoring proliferation at different time points, hence making it a strong and versatile tool.
References
- Panwar, J. & Roy, R. Integrated Field’s metal microelectrodes based microfluidic impedance cytometry for cell-in-droplet quantification. Microelectron. Eng. 215, 111010 (2019).
- Zhou, Y. et al. Characterizing Deformability and Electrical Impedance of Cancer Cells in a Microfluidic Device. Anal. Chem. 90, 912–919 (2018).
- Cahill, B. P. Optimization of an impedance sensor for droplet-based microfluidic systems. Smart Sensors, Actuators, MEMS V 8066, 80660F (2011).
Expertises & Resources
-
Expert Reviews: Basics of Microfluidics Pressure-Controlled Microfluidics in Organ-On-A-Chip Research Read more
-
Expert Reviews: Basics of Microfluidics Why Control Shear Stress in Cell Biology? Read more
-
Expert Reviews: Basics of Microfluidics How to choose a microfluidic chip Read more
-
Microfluidics White Papers An exploration of Microfluidic technology and fluid handling Read more
-
Expert Reviews: Basics of Microfluidics Passive and active mechanical stimulation in microfluidic systems Read more