PLGA microcapsules synthesis
In this application note, shell/aqueous core PLGA microcapsules are obtained using the Raydrop Double Emulsion developed and manufactured by Secoya, a capillary based microfluidic device equipped with a 3D printed injection nozzle simplifying the generation of double emulsion when used in combination with pressure based flow controllers.
The influence of the fluidic parameters on the PLGA microcapsule size and release from the oil across the shell are explored in this application note.
Secoya developed and manufactured the RayDrop used to perform this application note.

Introduction to PLGA microcapsules
What is the need?
Encapsulation of Active Pharmaceutical Compounds in core-shell microcapsules is of great interest for several purposes including: taste and odor masking as well as controlled release of drugs. In pharmaceutics, the possibility to encapsulate drugs, nutrients, and living cells that can be protected by a solid biocompatible shell can be used to target a specific site for therapy. [1]
What are the advantages of making double emulsion with PLGA shell?
In this context, PLGA microcapsules with shell and aqueous core have been widely studied because these polymer emulsions appear to be successful new drug delivery systems (DDS). Due to the good biocompatibility and biodegradability of this polymer, PLGA microcapsules can be used in various applications such as long-term drug release systems, vaccine adjuvants, and tissue engineering [2].
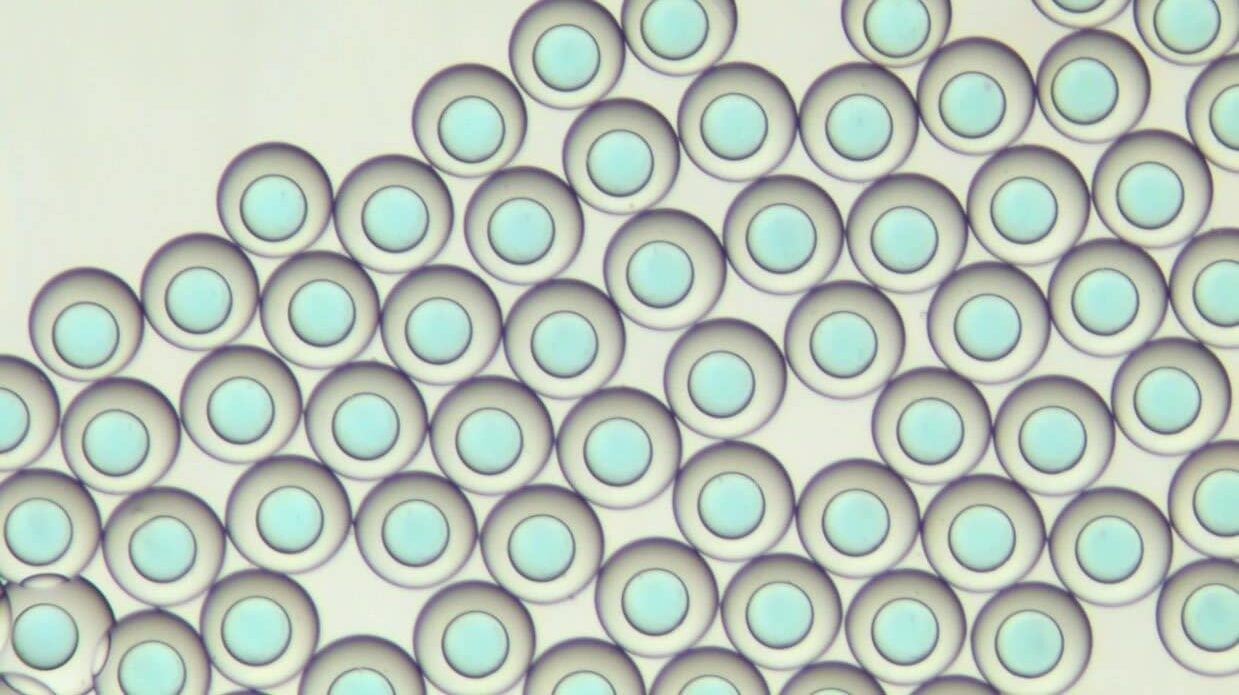
How to generate PLGA Microcapsules using microfluidics
PLGA Microcapsules System Setup
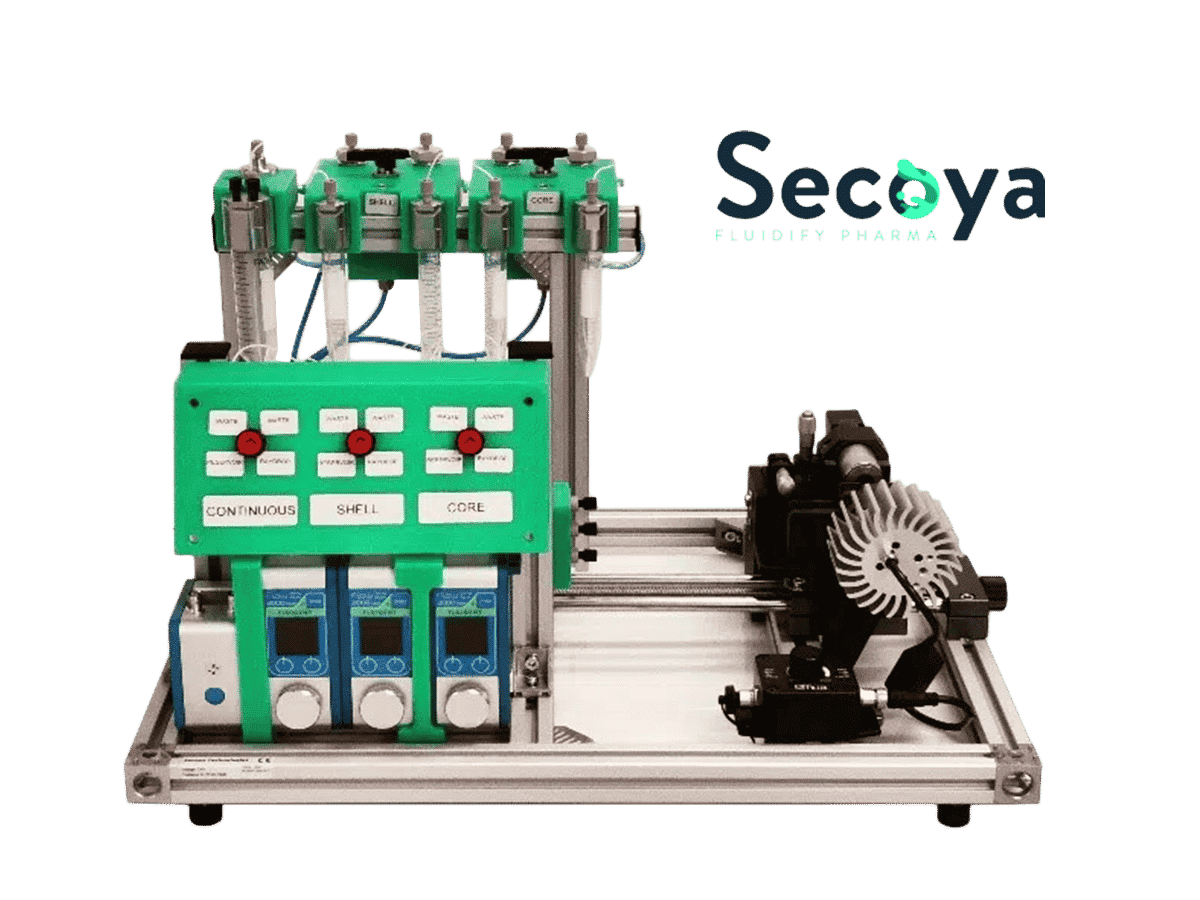
The production of droplets has been performed with the Complex Emulsions Production Platform, a lab system integrating all the components needed to produce simple and double emulsions.
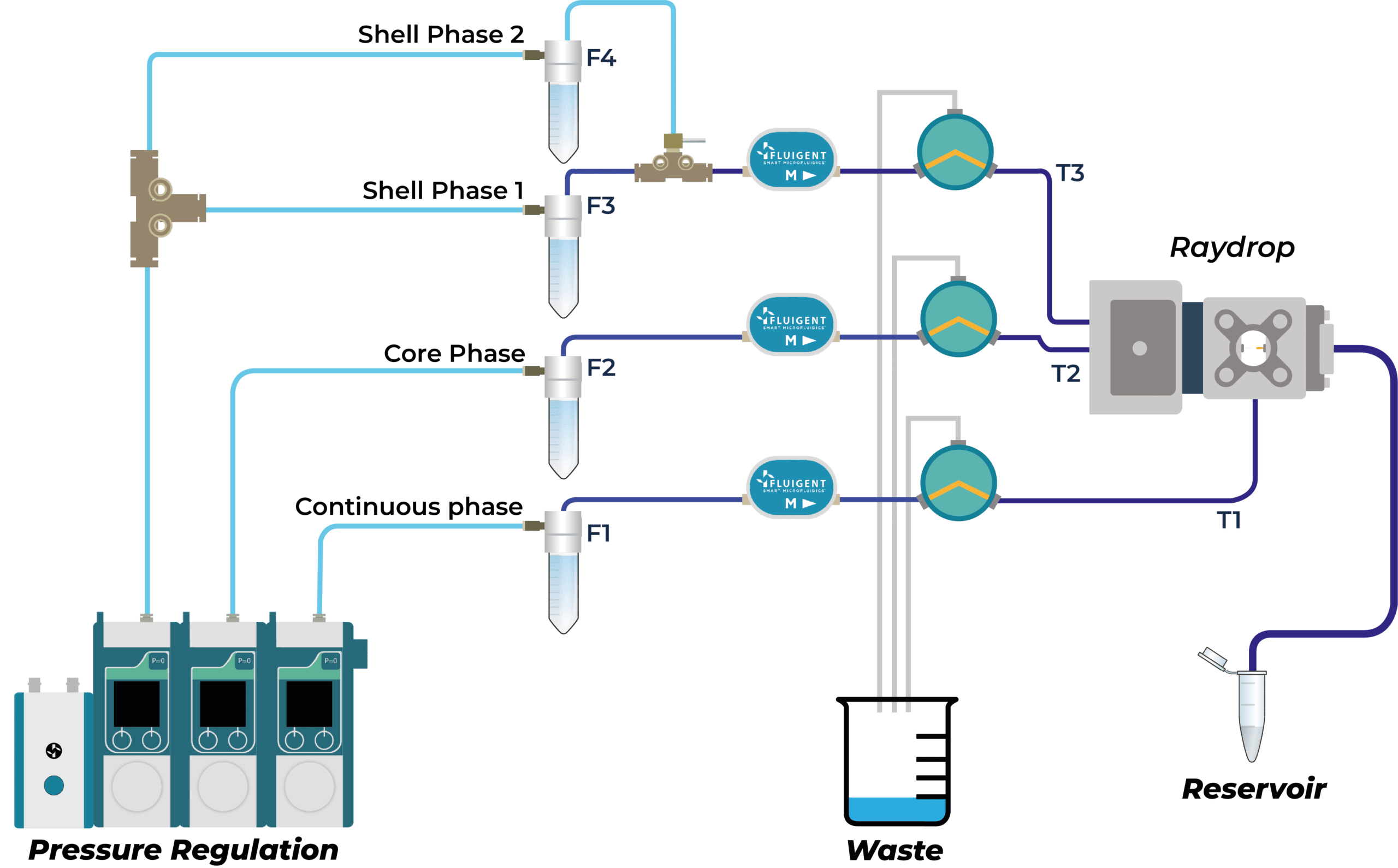
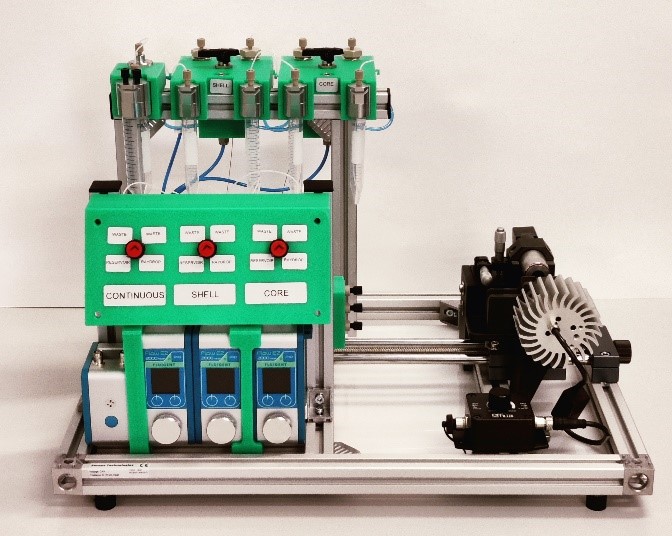
System component
Reagents
Core phase (and collect phase):
- Phosphate Buffered Saline buffer (PBS, pH=7,28 Sigma-Aldrich) containing blue food dye
Shell Phase 1: Priming and Cleaning Phase
- Ethyl acetate (EtOAc, Merck)
OR
- Isopropyl acetate (IPAc, Sigma-Aldrich)
Shell phase 2:
- Ethyl acetate (EtOAc, Merck) containing 10% Poly(D,L-lactide-co-glycolide) (PLGA, Resomer® RG 7555S ester terminated, Sigma-Aldrich)
OR
- Isopropyl acetate (IPAc, Sigma-Aldrich) containing 10% Poly(D,L-lactide-co-glycolide) (PLGA, Resomer® RG 7555S ester terminated, Sigma-Aldrich)
Continuous phase:
- Water containing 1% Poly(vinyl alcohol) (PVA, Sigma-Aldrich)
Synthesis of PLGA Capsules
Monodisperse double emulsion with PLGA shell synthesis is performed in 2 main steps:
- Generation of monodisperse double emulsion in the Raydrop
- Capsule formation by precipitation of the PLGA shell
1. Droplet Generation
To generate polymer emulsions easily, the system must be started with pure solvent in the shell phase (here IPAC). Once droplet formation is stabilized, the shell phase is switched to the solution containing the PLGA. This avoids possible clogging issues during the transient phase.
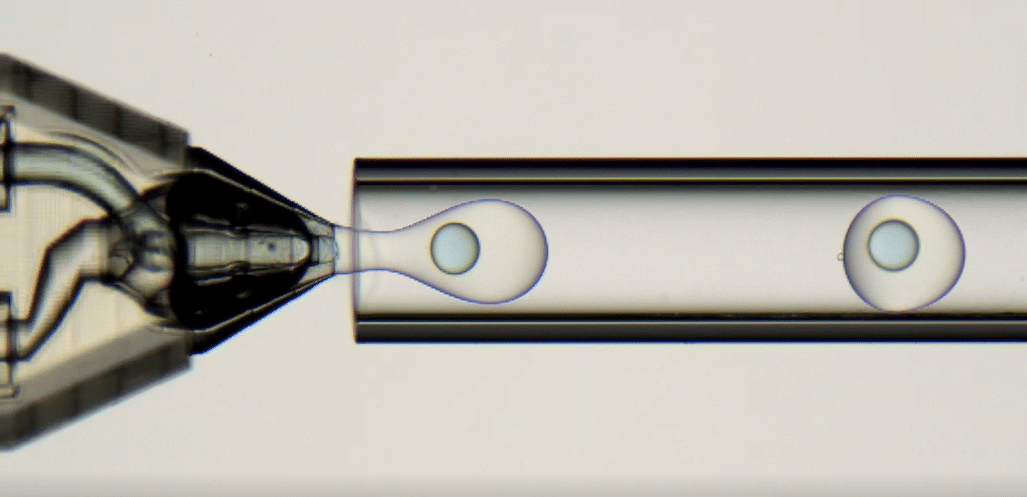

2. PLGA Microcapsule Formation
After being generated, the polymer emulsions are collected in a glass Petri dish. IPAc contained in the shell phase diffuses into the continuous phase so the PLGA precipitates. As a result, droplets are solidified and become PLGA microcapsules.
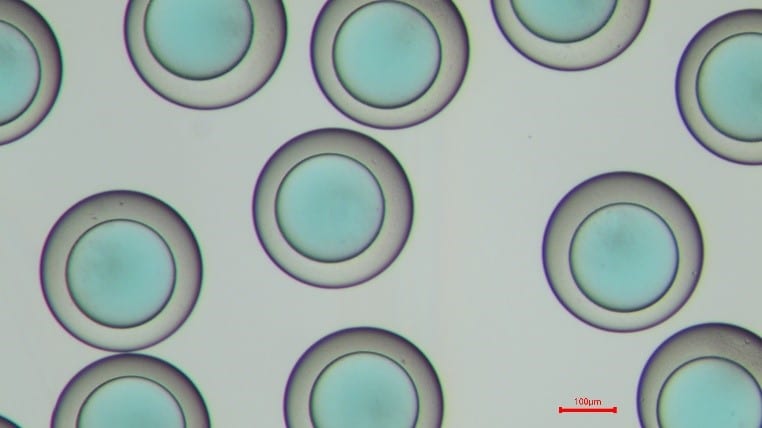
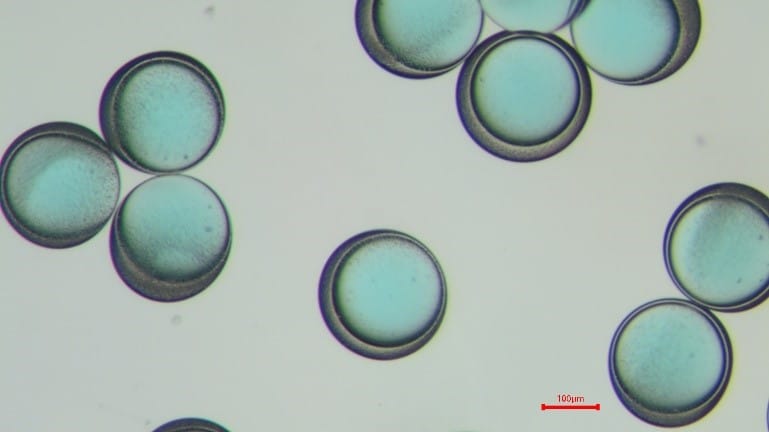
Figure 6: PLGA microcapsules in the PBS solution. The left shows 15 seconds after the creation in the Raydrop. The right displays 200s in the PBS solution after the creation in the Raydrop. The shell thickness decreases, as the IPAc contained in the shell phase diffuses in the continuous phase.
To go further
Partial Results: PLGA Microcapsules Synthesis
In this application note, different parameters have been studied. Firstly, the evolution of the PLGA microcapsules over time have been observed. Then, the release rate of the microcapsules was measured.
Evolution of the Droplet Diameter During the Precipitation Process
Once formed, the droplets are collected in the same solution used in the core of droplets to match the osmolarity of inner and outer aqueous phases. An analysis of the size of the PLGA microcapsules is performed using a microscope and measurement software.
For a given sample, several measurements of the microcapsule diameter are made at different times. The evolution of the diameter is highlighted in Figure 7 and the operating conditions are shown in Table 1.
| Continuous phase | Shell | Core | |
| Composition | Water + 1% PVA | EtOAc + 10% PLGA | PBS pH = 7,28 + dye |
| Pressure (mbar) | 214 | 2404 | 104 |
| Flow rate (µL/min) | 109 | 16.5 | 9.1 |
Table 1: Operating conditions for the evaluation of the diameter of capsules
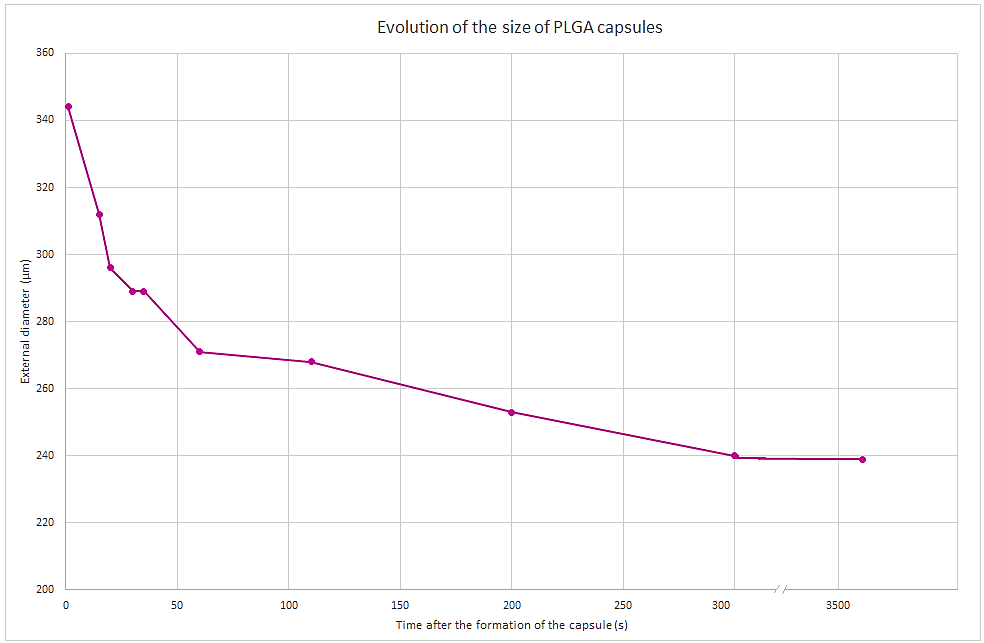
Double emulsions with PLGA shell have a diameter ranging from 312 µm to 230 µm. We observe that during the precipitation process, the diameter of the microcapsules decreases. A diameter of 312 µm is obtained 20 seconds after droplet formation, while a diameter of 230 µm is obtained 300 seconds after droplet formation. After 300s, we observed that microcapsules are reaching a steady state and do not decrease further.
Conclusion
The production of stable monodispersed microcapsules with a solid PLGA shell and an aqueous core using a microfluidic platform has been successfully achieved. The microfluidic platform allows one to optimize not only the core diameter but also vary the shell thickness by adjusting the flow rates of the different fluids.
These PLGA microcapsules can be used in a wide range of applications, like the encapsulation of active ingredients such as specific drugs, which will be delivered according to the pH acidity [3].
Related Resources
-
Expert Reviews: Basics of Microfluidics 10 Tips for Reliable Droplet Generation Read more
-
Microfluidic Application Notes 1-10 microns PLGA microsphere production using the RayDrop Read more
-
Microfluidics White Papers Double emulsion for the generation of microcapsules – a Review Read more
-
Microfluidic Application Notes Encapsulation of Cells In Small Double Emulsions Read more
-
Microfluidic Application Notes Alginate Microcapsule Synthesis Read more
-
Microfluidic Application Notes PLGA nanoparticle synthesis using 3D microfluidic hydrodynamic focusing Read more
-
Microfluidics White Papers Droplet-based Microfluidics – A Complete Guide Read more
-
Microfluidic Application Notes PLGA Microparticles Synthesis Read more
Webinar Recording
WEBINAR: Encapsulation of fluorescent bacteria in double emulsions for FACS sorting
Discover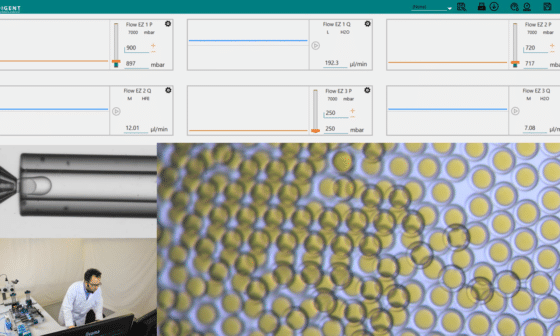
WEBINAR – Master the production of Double Emulsions
Discover
Webinar Complex Microfluidic Emulsions: How to Optimize Production, Flow Control & Monodispersity
Discover
Related Products
References
[1] LEE, Myung Han, HRIBAR, Kolin C., BRUGAROLAS, Teresa, KAMAT, Neha P., BURDICK, Jason A. and LEE, Daeyeon, 2012. Harnessing Interfacial Phenomena to Program the Release Properties of Hollow Microcapsules. Advanced Functional Materials. 11 January 2012. Vol. 22, no. 1, p. 131–138. DOI 10.1002/adfm.201101303.
[2] Qi, F., Wu, J., Li, H., & Ma, G. (2018). Recent research and development of PLGA / PLA
microspheres / nanoparticles: A review in scientific and industrial aspects
[3] MONTAZERI, Leila, BONAKDAR, Shahin, TAGHIPOUR, Mojtaba, RENAUD, Philippe and BAHARVAND, Hossein, 2016. Modification of PDMS to fabricate PLGA microparticles by a double emulsion method in a single microfluidic device. Lab on a Chip. 2016. Vol. 16, no. 14, p. 2596–2600. DOI 10.1039/C6LC00437G.
[4] TU, Fuquan and LEE, Daeyeon, 2012. Controlling the Stability and Size of Double-Emulsion-Templated Poly(lactic- co -glycolic) Acid Microcapsules. Langmuir. 3 July 2012. Vol. 28, no. 26, p. 9944–9952. DOI 10.1021/la301498f.