The Role of Microfluidics in Advanced Organoid Modeling: from Static to Dynamic
What are Organoid Models?
Organoids are small, three-dimensional (3D) multicellular structures derived from stem cells that self-organize into miniaturized, simplified versions of organs. Organoid modeling simulates key aspects of organ development, architecture, and function. Compared to conventional cell culture, organoids mimic organ-specific cell heterogeneity, spatial organization, and functional outputs, making them powerful tools for studying disease mechanisms, host-pathogen interactions, and personalized medicine.
Organoids have been developed for a wide range of tissues and organs, including the brain, retina, liver, lung, gut, kidney, pancreas, and various tumors. Each organoid type exhibits specific tissue-like features. Intestinal organoids can form crypt–villus structures, retinal organoids develop layered photoreceptor arrays, and kidney organoids produce nephron-like tubules. These models have provided insights into human biology, especially where access to human or animal tissue is limited or ethically constrained.
Despite their high biological fidelity, conventional static organoid cultures present some limitations. They are often embedded in extracellular matrix (ECM) gels like Matrigel or grown in suspension. This can restrict nutrient and oxygen diffusion and lead to the formation of necrotic cores in larger organoids. This diffusion barrier limits size, longevity, and cellular maturation. Static systems also do not replicate dynamic physical cues, such as fluid shear from perfusion that is essential in cell maturation and differentiation. These shortcomings hinder the modeling of vascularized tissues and complex organ-level functions, reducing the translational relevance of the systems for drug screening or disease modeling.
Fundamentals: Microfluidics Meets Organoids
Microfluidic technology overcomes these barriers by precisely manipulating fluids at the microscale under laminar flow. Such systems provide a high level of control over the cellular microenvironment. This reproducibly enables continuous perfusion of nutrients and oxygen, the application of mechanical stimuli, and the establishment of biochemical gradients. As a result, microfluidic platforms enhance organoid viability, promote tissue maturation, and support vascularization.
Operating with small fluid volumes, microfluidics enables real-time monitoring, gradient generation, and precise delivery of drugs and signaling molecules. It is possible to perform high-throughput screening and single-organoid resolution in a microfluidic device, offering greater scalability and experimental control.
Several microfluidic modalities are used in organoid research:
Closed-channel systems: mimic vasculature with perfusable channels. Fig. 1 demonstrates how organoids can be perfused in the middle channel connected to the inlet and outlet, while the adjacent channel is loaded with endothelial cells and fibroblasts embedded in the hydrogel to promote the vascularization of the tumoroid.(1)
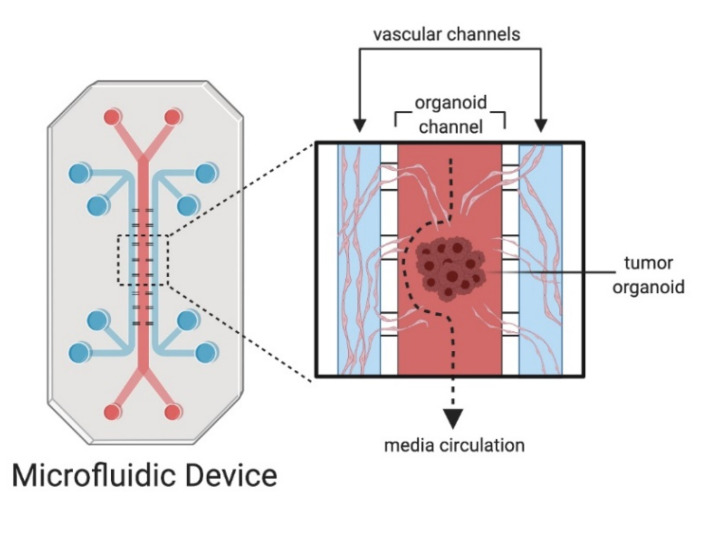
Figure 1: Microfluidic devices and organoids on chips. The tumoroid culture is perfused with vasculature to model angiogenesis.
- Open microfluidics: enable easier access to the tissue for analysis. As shown in Fig 2, a 3D-printed microfluidic device was designed to host early neural organoids, allowing vascularization from surrounding channels seeded with hPSC-derived vascular cells. This configuration can be sealed for perfusion culture and later unsealed to facilitate analytical procedures such as flow cytometry and proteomics.(2)
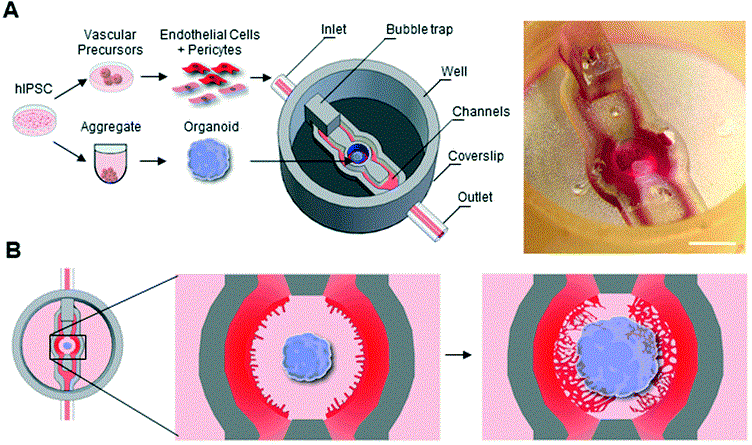
Figure 2: (A) Stereomicroscope image of the organoid on a chip. Scale: 2mm. (B) Schematic representations of on chip angiogenesis
- Droplet and microbead microfluidics: encapsulate cells in uniform droplets for rapid organoid generation. In the Fig.3 illustrates the use of microbead capsules for cell molecular analysis and high-throughput handling. (3)
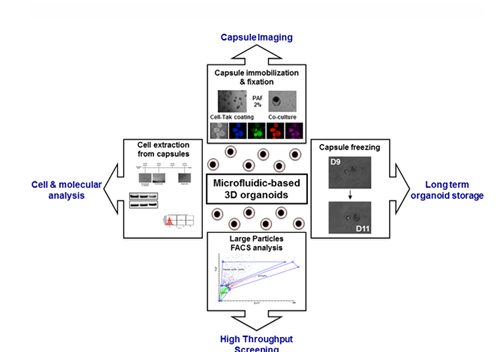
Figure 3: Methodological developments for microbead handling and studies.
Transition from Static to Dynamic: Technological Implementation
What are limitations of culturing organoids in static conditions?
Static organoid cultures are limited by size due to diffusion barriers, which is the maximum distance over which oxygen, nutrients, and metabolites can effectively diffuse into a 3D tissue structure (like an organoid) to support cell viability and function. Beyond this critical distance, cells experience hypoxia or nutrient starvation, leading to necrotic core formation. Usually organoids larger than 300-500µm in diameter develop necrotic core, particularly problematic in static culture, like Matrigel domes and suspensions. Furthermore, organoids lacking vasculature and mechanical cues fail to achieve physiological maturation and functional complexity.
Table 1. Diffusion Limits in Organoids and 3D Tissue Culture
| Molecule | Approximate Diffusion Limit in 3D Tissue | Biological Implication | Reference |
|---|---|---|---|
| Oxygen (O₂) | ~100–200 μm | Hypoxia and cell death beyond the limit | (4) |
| Nutrients (e.g., glucose) | ~200–400 μm | Energy deficit, impaired proliferation and proliferation | (5) |
| Waste remova | ~200–400 μm | Accumulation of toxic byproducts can impair function. | (5) |
1. Perfusion of Organoid Models and Vascularization
Perfusion-based microfluidic platforms advance organoid culture beyond the limitations of static systems. By providing continuous, controlled fluid flow, these platforms may be used for vascularization, enhancing nutrient and oxygen delivery and applying shear stress that supports tissue maturation.
Learn here more about perfusion techniques and use cases.
A key example is where the perfusion of kidney organoids led to significantly enhanced vascularization and tissue development. Under controlled high fluid shear stress (1-4.27 mL/min), kidney organoids exhibited a fivefold increase in vascular area (PECAM1 transcripts), a tenfold increase in vessel branching, and elevated expression of endothelial genes. These outcomes surpassed those achieved under static conditions (Fig 4.). In this model organoid was immobilized in the channel and perfused in the closed channel (6).
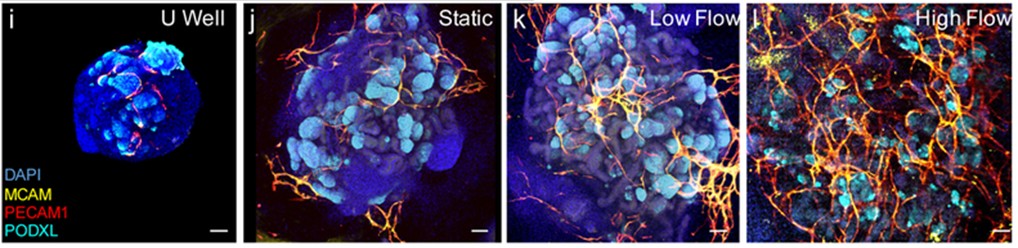
Figure 4: Confocal 3D image of vascular markers in whole-mount organoids cultures under static, low and high fluid shear. Scale bards =100µm.
Another approach to organoid vascularization involves embedding perfusable components within the chip. In a vascularized kidney organoid-on-chip model, separate channels were designed for endothelial and organoid compartments. This configuration allowed endothelial cells to form functional connections with organoid vasculature, enabling molecular exchange, cell migration and structural integration(7)
Explore the co-culture of kidney organoids with HUVECs here.
A broader review confirms that vascularization is essential for organoids to exceed several hundred microns in size and to reach advanced developmental stages. Without vascular support, diffusion limitations result in central necrosis, limited cellular diversity, and immature tissue architecture (8). Likewise, recent work highlights the development of “organoid-on-chip” (OOCoid) systems that incorporate perfusable vasculature and fluidic forces to support long-term viability and physiological function across various models, including lung, brain, kidney, and tumor tissues(9).
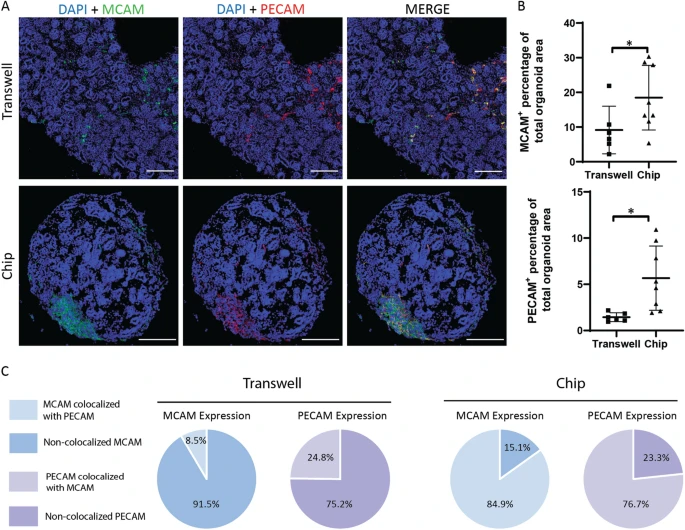
Figure 5: Immunofluorescent images of DAPI-MCAM-PECAM co-staining showing kidney organoids cultured on transwell and on chip, scale bars = 200 µm. (B) Statistical analysis of MCAM and PECAM expression in kidney organoids based on percentage of total area.
2. Dynamic Culture of Organoids for Maturation and Differentiation
Mechanical cues such as shear stress, hydrostatic pressure, and cyclic tension are recognized as regulators of organoid development and function. Cells detect and respond to these mechanical forces through mechanotransduction pathways that influence gene expression and tissue organization (10). A well-characterized aspect of mechanotransduction involves the interaction of cells with the physical properties of the surrounding extracellular matrix (ECM), including matrix stiffness and the presence of adhesive ligands—both of which have direct effects on stem cell fate and lineage commitment.
Standardization of these properties remains challenging due to batch-to-batch variability in biological ECMs such as Matrigel, which affects both protein composition and mechanical stiffness. This variability complicates reproducibility and scalability in organoid research (11).
Beyond the matrix itself, mechanical stresses imparted by the culture environment, especially the mode and parameters of perfusion, are critical determinants of organoid maturation. Midbrain organoids cultured under continuous, controlled laminar flow exhibited enhanced differentiation into dopaminergic neurons and significantly reduced necrotic core formation compared to those grown under orbital shaking (12). This suggests that the dynamic mechanical environment is essential for achieving physiologically relevant organoid models.
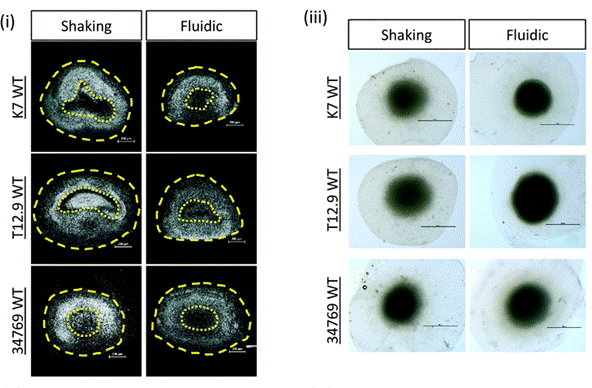
Figure 6: Hoeschst staining of nuclei (white) pf representative human Midbrain Organoid (hMO) sections derived from three different WT NESC lines and culture shaking or fluidic conditions. Yellow dotted line indicates the area of “dead core” (scale bar = 200 μm)
3. Organoids Encapsulation for High-throughput Homogeneous Culturing
Droplet microfluidics, using pressure-based control systems (encapsulation kit link), enables the encapsulation of cells into uniform nanoliter-scale droplets. These systems can be used to produce organoid units, that are critical for comparative studies and drug screening. As an example, microbead-based droplet platforms permit embedding of extracellular matrix components within droplets, enhancing 3D culture fidelity and cell-matrix interactions (13).
Read the prostate organoids in microbeads review.
Read the application note on the encapsulation of cells in small double emulsions.
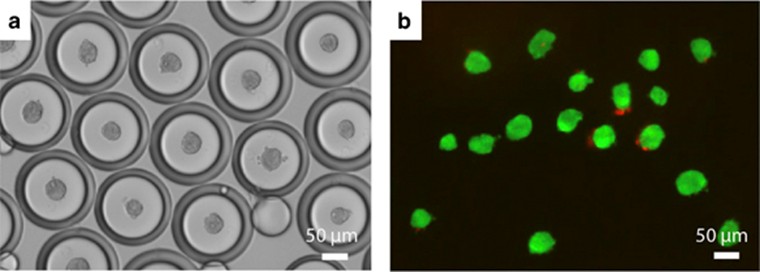
Figure 7: (a) Phase image of human mesenchymal stem cells (hMSC) spheroids encapsulated in double emulsion droplets after 6 h. (b) Live/dead staining of spheroids after release from emulsion at 6 h using 1H,1H,2H,2H-perfluoro-1-octanol. Live cells were labeled with calcein AM (green), and dead cells were labeled with propidium iodide (red). (13)
Summary and Future Directions
The integration of controlled flow into organoid research marks a pivotal advancement in overcoming the long-standing limitations of static 3D cultures. Traditional organoid systems are constrained by diffusion barriers. This results in poorly controlled biochemical microenvironments, and the absence of biomechanical cues. Both hinder tissue maturation and scalability.
Closed-channel microfluidic platforms, droplet-based encapsulation systems, and microbead workflows, when paired with precise flow control, enable tight environmental control and fine-tuned manipulation of mechanical forces. These features are pivotal for modeling complex organ development, inducing vascularization, and guiding region-specific cell fate decisions. Mechanical stimuli such as shear stress and pressure are now proven to significantly impact organoid structure, viability, and functional differentiation, especially in brain, kidney, and vascular models.
Looking forward, future directions will focus on the integration of additional dynamic parameters, such as spatiotemporal signaling gradients, within microfluidic systems to further mimic in vivo organogenesis.
The combination of organoid-on-chip systems with real-time biosensors and modular platforms for multi-organ interfacing (body-on-chip) is expected to enhance the translational potential of organoids for disease modeling, drug discovery, and personalized medicine. Continued efforts in standardizing ECM materials and automating droplet microfluidics will be crucial for reproducibility and scalability in clinical and industrial applications.
Related Products
Related Expertises
-
Microfluidics Case Studies Micropipette Aspiration of Red Blood Cells Mechanosensitivity and Mechanics Read more
-
Microfluidics Case Studies Gut-on-Chip Modeling: From Chip Development to Perfusion Read more
-
Expert Reviews: Basics of Microfluidics Optimizing Microfluidic Perfusion: Best Practices and Innovations Read more
-
Microfluidics Case Studies Creating kidney organoids‑vasculature interaction model using Fluigent’s Flow-EZ Read more
-
Expert Reviews: Basics of Microfluidics Pressure-Controlled Microfluidics in Organ-On-A-Chip Research Read more
-
Microfluidics White Papers A review of Organ on Chip Technology – A White Paper Read more
-
Expert Reviews: Basics of Microfluidics Why Control Shear Stress in Cell Biology? Read more
-
Expert Reviews: Basics of Microfluidics Micropipette aspiration of cells and tissues Read more
-
Microfluidic Application Notes Peristaltic Pump vs Pressure-Based Microfluidic Flow Control for Organ on Chip applications Read more
-
Microfluidic Application Notes Droplet Sequencing: Drop-Seq method Read more
-
Expert Reviews: Basics of Microfluidics Prostate Organoid Culture in Microbeads Read more
References
1. Gunti S, Hoke ATK, Vu KP, London NR. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers. 2021 Jan;13(4):874.
2. Salmon I, Grebenyuk S, Fattah ARA, Rustandi G, Pilkington T, Verfaillie C, et al. Engineering neurovascular organoids with 3D printed microfluidic chips. Lab Chip. 2022;22(8):1615–29.
3. Laperrousaz B, Porte S, Gerbaud S, Härmä V, Kermarrec F, Hourtane V, et al. Direct transfection of clonal organoids in Matrigel microbeads: a promising approach toward organoid-based genetic screens. Nucleic Acids Res. 2018 July 6;46(12):e70.
4. Ziółkowska-Suchanek I. Mimicking Tumor Hypoxia in Non-Small Cell Lung Cancer Employing Three-Dimensional In Vitro Models. Cells. 2021 Jan;10(1):141.
5. Kim D, Kim W, Sharma H, Lee S, Park C, Park S, et al. Ultra-Tiny Gelatin Nanoparticles-Assisted 3D Stem Cell Spheroids for Engineering Tissue Regeneration. Adv Healthc Mater. n/a(n/a):2501882.
6. Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods. 2019 Mar;16(3):255–62.
7. Bas-Cristóbal Menéndez A, Du Z, van den Bosch TPP, Othman A, Gaio N, Silvestri C, et al. Creating a kidney organoid-vasculature interaction model using a novel organ-on-chip system. Sci Rep. 2022 Nov 30;12(1):20699.
8. Zhang S, Wan Z, Kamm RD. Vascularized organoids on a chip: strategies for engineering organoids with functional vasculature. Lab Chip. 2021 Feb 9;21(3):473–88.
9. Wang X, Bijonowski BM, Kurniawan NA. Vascularizing Organoids to Promote Long-Term Organogenesis on a Chip. Organoids. 2023 Dec;2(4):239–55.
10. Morena F, Armentano I, Montanucci P, Argentati C, Fortunati E, Montesano S, et al. Design of a nanocomposite substrate inducing adult stem cell assembly and progression toward an Epiblast-like or Primitive Endoderm-like phenotype via mechanotransduction. Biomaterials. 2017 Nov 1;144:211–29.
11. Taghizadeh M, Taghizadeh A, Kim HS. Mechanobiological engineering strategies for organoid culture. APL Bioeng. 2025 July 18;9(3):031501.
12. Berger E, Magliaro C, Paczia N, Monzel AS, Antony P, Linster CL, et al. Millifluidic culture improves human midbrain organoid vitality and differentiation. Lab Chip. 2018 Oct 9;18(20):3172–83.
13. (PDF) One drop at a time: Toward droplet microfluidics as a versatile tool for single-cell analysis. ResearchGate [Internet]. [cited 2025 Aug 8]; Available from: https://www.researchgate.net/publication/278401605_One_drop_at_a_time_Toward_droplet_microfluidics_as_a_versatile_tool_for_single-cell_analysis


