University of Cambridge: Microfluidic GUV production and testing
In the pursuit of Giant Unilamellar Vesicle (GUV) production, Fluigent pressure-based flow controllers have played a crucial role in fluid control during experimentation. The microfluidics-based approach known as Octanol-Assisted Liposome Assembly (OLA), complemented by precise pressure control, has emerged as a powerful method in these studies.
The use of Fluigent pressure-based flow controllers has been a key factor in achieving microfluidic GUV production using the OLA method, facilitating the production of vesicles with higher monodispersity and size control.
About The Keyser lab – University of Cambridge
The Keyser Lab is a group of researchers at the Cavendish Laboratory, University of Cambridge, UK. Since its founding in 1874, the Cavendish Laboratory has been at the forefront of discovery in physics, with a core focus on experimental physics supported by excellence in theory. The department promotes world-leading experimental and theoretical physics in all its diversity. Scientists from the Keyser Lab study the physics of ions, macromolecules and particles, with a particular focus on particles in confined geometries at the single molecule/particle level. To exert maximum control over all parameters for their experiments, they make use of several cutting-edge techniques such as DNA self-assembly (origami), optical trapping, electrophysiology, and microfluidics and nanofluidics.
The team includes researchers with expertise in physics, engineering, physical chemistry, biochemistry/biology, and micro- and nanofabrication.

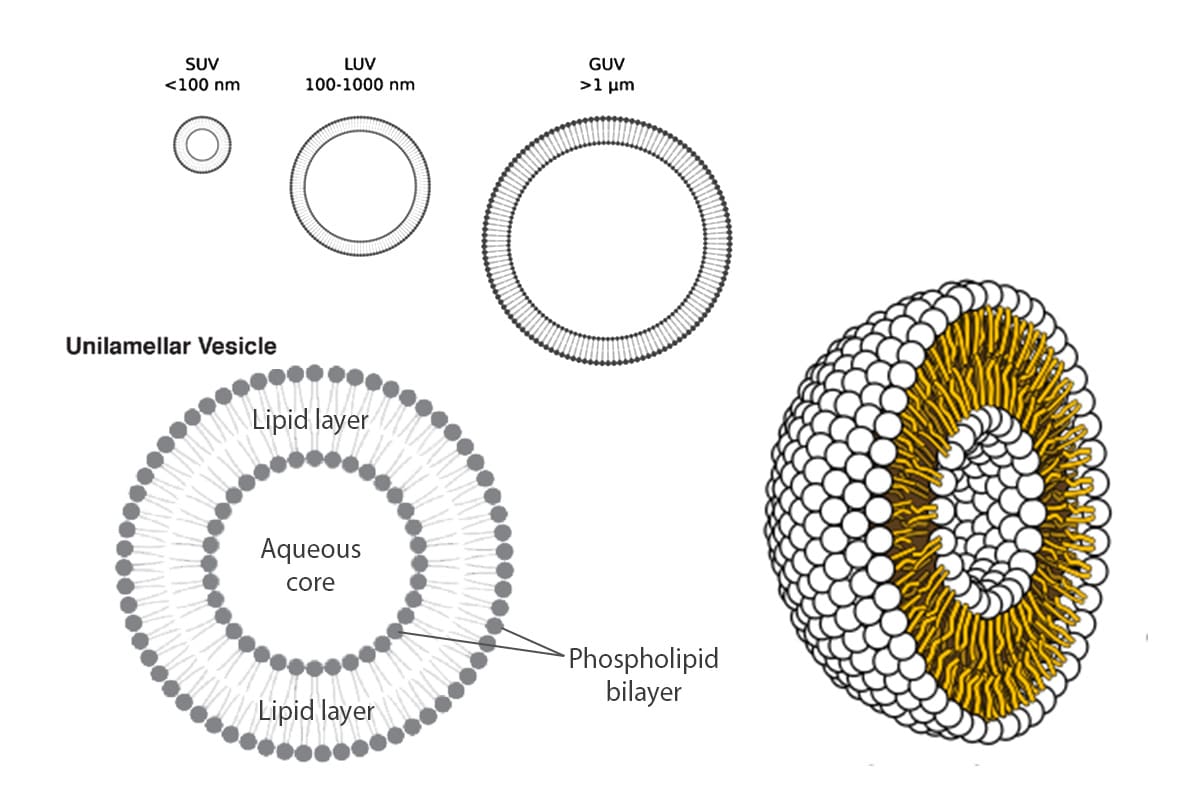
What are Giant Unilamellar Vesicles (GUVs)?
Giant Unilamellar Vesicles (GUVs) are micron-sized compartments composed of lipid bilayers, serving as models for cell membranes or as encapsulating structures for biological materials within cell-like environments. These vesicles have dimensions ranging from 1 to 100 µm, mimicking the size of cells. Like natural cell membranes, GUVs are constructed from lipids, predominantly phospholipids and cholesterol. The amphiphilic properties of these lipids enable them to spontaneously arrange into spherical compartments when immersed in an aqueous solution.
They offer the advantages of having well-defined lipid compositions, being easy to image, and being controlled systems for studying transport processes. These characteristics open the door to a variety of applications in biology and biomedicine, especially membrane biophysics and synthetic biology.1,2
Working principle of GUV production
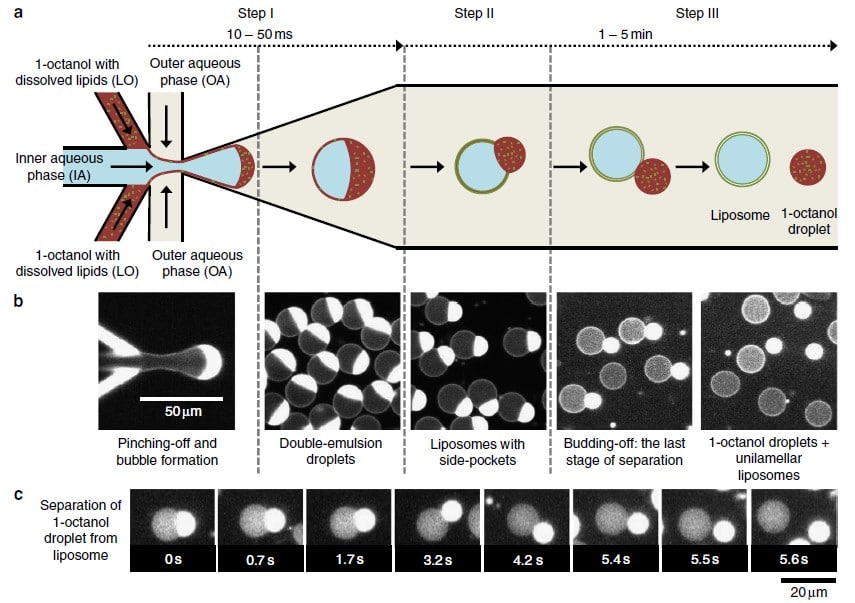
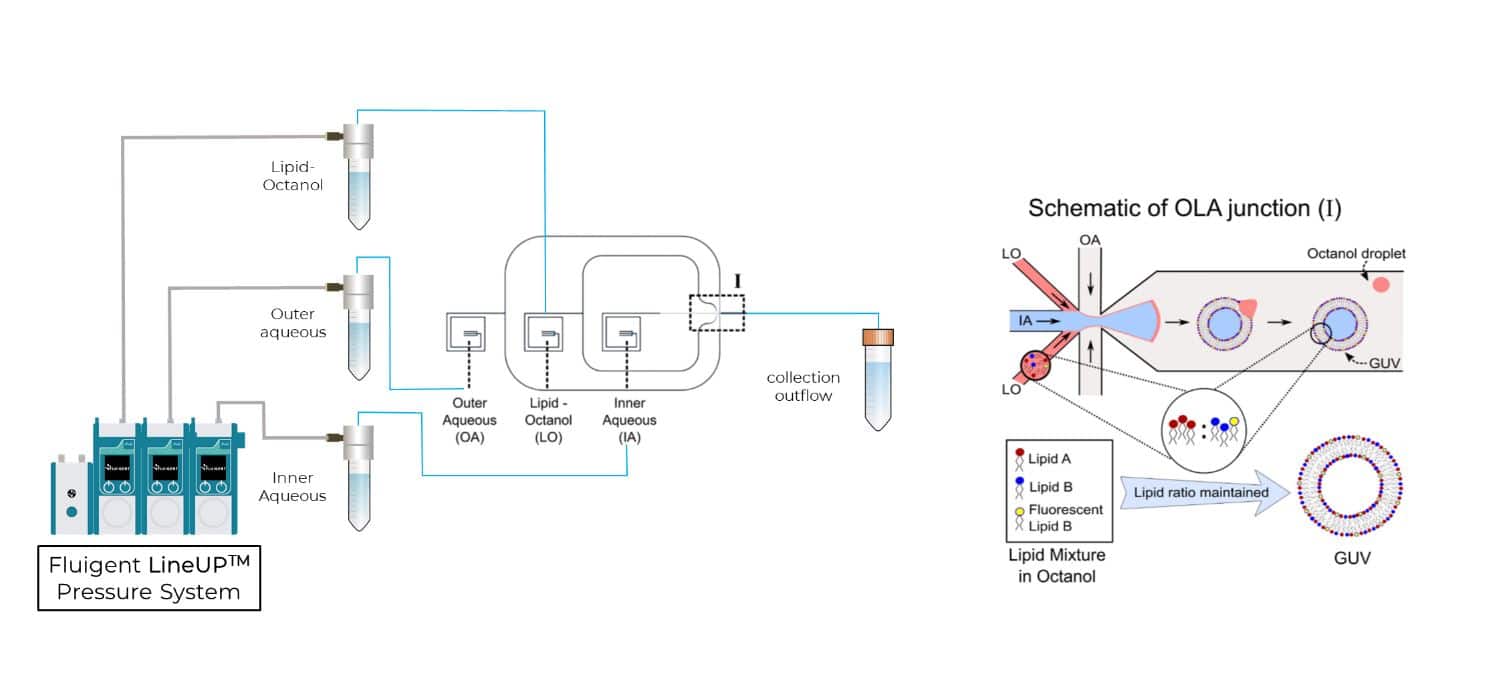
In 2016, the Dekker Laboratory from the Delft University of Technology developed a novel microfluidics-based method called Octanol-Assisted Liposome Assembly (OLA) to create uniform, cell-sized (5–20 mm) GUVs with high encapsulation efficiency. Using Fluigent’s pressure-driven MFCS-EZ controller with the Oxygen software tool, an inner aqueous phase (IA) and an outer lipid-carrying 1-octanol phase (LO) were combined, resulting in double-emulsion droplets via hydrodynamic flow focusing. These droplets developed a side-connected 1-octanol pocket, which, due to interfacial energy minimization, separated to rapidly form fully assembled solvent-free liposomes. Microfluidic GUV production addresses the persistent issue of residual oil in vesicle bilayers.
This method allows researchers to generate GUVs with higher monodispersity and greater size control compared to traditional methods, and with a faster process than alternative microfluidic methods.3
Keyser Lab: Microfluidics for GUV generation for antimicrobial efficacy and biomimetic vesicle membrane testing
Scientists from the University of Cambridge integrated octanol-assisted liposome assembly into their microfluidic platforms (“lab on a chip” devices) for quantifying drug permeation and antimicrobial efficacy on biomimetic vesicle membranes.
In a first paper published in Lab on a Chip (2019), they reported on a microfluidic platform for testing antimicrobial peptides on artificial vesicle membranes. This platform produced vesicles with an encapsulated dye to assess the efficacy of antimicrobial peptides by measuring the time it takes for vesicles to lyse.4
This Giant Unilamellar Vesicle production technique also utilized Fluigent‘s MFCS-EZ and its accompanying software (Oxygen) for fluid control. Vesicle formation and perfusion inlets were connected, and the microfluidic chip was loaded with an inner aqueous phase base stock. The outlet was connected to Fluigent’s 2-Switch, which can switch between open or closed configurations for waste removal. Vesicles flowed through a channel to the connector chip, and 1-octanol pockets pinched off to form droplets. Density-based separation removed vesicle production waste, and the vesicles were distributed to trapping chambers. Peptide doses were controlled, replacing the inner aqueous phase buffer, while maintaining a constant input pressure. The entire process was monitored under an inverted microscope.
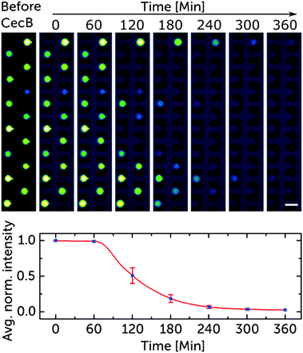
Validated with cecropin B on bacterial-mimicking membranes, the platform enabled the researchers to study over 1000 vesicles simultaneously. The results showed dose-dependent disruption of vesicle membranes, demonstrating the platform’s potential for controlled, quantitative assessments of the efficacy and selectivity of antimicrobial peptides. This microfluidic approach to GUV production is suggested as a new standard for pre-clinical development of membrane-active antimicrobials, offering advantages in cost efficiency and parallelization.
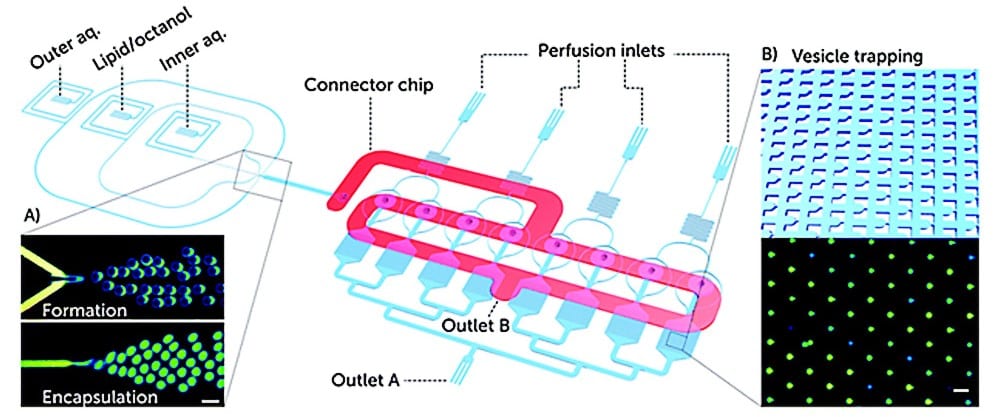
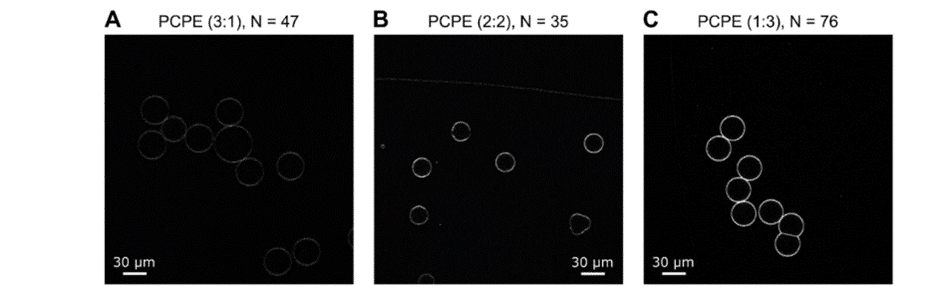
In another paper published in Biomembranes (2020), scientists from the University of Cambridge and the University of Exeter produced GUVs with tunable binary lipid mixtures to determine lipid diffusion in OLA vesicles.5
The focus was on expanding the capabilities of this microfluidic approach to form GUVs with tunable binary lipid mixtures. An MFCS-EZ equipped with a Fluiwell-4C reservoir kit with OLA solutions was connected to the microfluidic chip. The microfluidic GUV production method allowed for a high degree of control over vesicle sizes by adjusting pressures in different channels. This was possible due to the high responsiveness, stability and repeatability of the pressure generated by the MFCS-EZ. In addition, flow speed was successfully matched to the outlet channel length to prevent residual octanol attachment to vesicles.
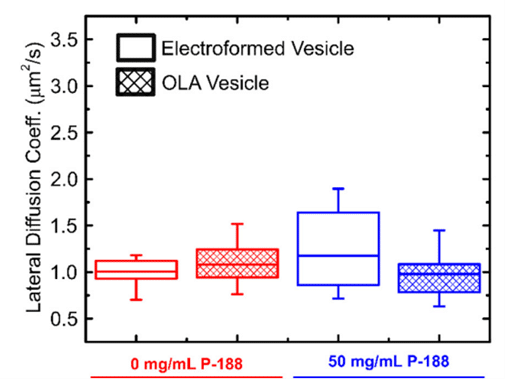
This study employed fluorescence recovery after photobleaching to investigate lipid lateral diffusion coefficients in GUVs produced by the microfluidic approach, finding values within the expected range. Comparisons with electroformed vesicles indicated quantitative similarity in lipid diffusion coefficients. The results served as a quantitative biophysical validation of OLA-derived GUVs, enhancing the potential application of this versatile platform in drug discovery, artificial cell production, and lipid membrane studies.
Conclusion
To produce Giant Unilamellar Vesicles, fluids were controlled using Fluigent pressure-based flow controllers. Researchers from the Keyser Lab (University of Cambridge) typically operated the chip with input pressures of 40 mbar for the inner aqueous phase and dissolved-lipid phases and 100 mbar for the outer aqueous phase. For precise flow measurements, Fluigent Flow Units can be added on the fluidic path. Microfluidic GUV production also allowed researchers to adjust the sizes of the generated vesicles by adjusting the microfluidic pressures of the phases. This degree of control is difficult to achieve using standard methods such as electroformation or traditional syringe pumps.

Testimonials

“Microfluidics presents various advantages to researchers who need small volumes and high throughput in answering their scientific questions. In our lab, we use microfluidic devices for standardization and control of experimental parameters like concentration and timing. In the complex (biological) systems we are working on, the mentioned characteristics are fundamental in collecting reliable meaningful statistics, and microfluidics in combination with light microscopy offers just that. We also heavily rely on the ability to rapidly prototype devices, as we can design bespoke solutions at minimal production cost and time.”
“We use the pressure-based pumps from Fluigent for experiments that require swift responsiveness when manipulating fluids, and fine tuning at low flow rates. We use the Fluigent systems during fabrication and running of the microfluidic chips. The ability to pump in air at high precision makes the Fluigent pressure-based systems ideally suited to selectively coat and functionalize micro-channels within a microfluidic network. After coating we then fill the devices with the experimental solutions and use the pressure controls to move fluids around, open and close valves and carefully time the introduction of small molecules in the experiments.“
Kareem Al Nahas, University of Cambridge
Expertises & Resources
-
Expert Reviews: Basics of Microfluidics Giant Unilamellar Vesicles (GUVs) Production using Microfluidics Read more
-
Microfluidics Article Reviews A mRNA encapsulation platform integrating Fluigent’s FlowEZ Read more
-
Expert Reviews: Basics of Microfluidics Microfluidics for vaccine development Read more
-
Microfluidic Application Notes Liposome Nanoparticle Synthesis Read more
-
Expert Reviews: Basics of Microfluidics Flow control for droplet generation using syringe pumps and pressure-based flow controllers Read more
References
- Naziris, N., Demetzos, C. (2021). Liposomes: Production Methods and Application in Alzheimer’s Disease. In: Vlamos, P. (eds) GeNeDis 2020. Advances in Experimental Medicine and Biology, vol 1339. Springer, Cham.
- Pereira, David & Valentão, Patrícia & Andrade, Paula. (2014). Nano- and Microdelivery Systems for Marine Bioactive Lipids. Marine Drugs. 12. 6014. 10.3390/md12126014.
- Deshpande, S.; Caspi, Y.; Meijering, A. E. C.; Dekker, C. Octanol-Assisted Liposome Assembly on Chip. Nat Commun 2016, 7 (1), 10447.
- Al Nahas, K.; Cama, J.; Schaich, M.; Hammond, K.; Deshpande, S.; Dekker, C.; Ryadnov, M. G.; Keyser, U. F. A Microfluidic Platform for the Characterisation of Membrane Active Antimicrobials. Lab Chip 2019, 19 (5), 837–844.
- Schaich, M.; Sobota, D.; Sleath, H.; Cama, J.; Keyser, U. F. Characterization of Lipid Composition and Diffusivity in OLA Generated Vesicles. Biochimica et Biophysica Acta (BBA) – Biomembranes 2020, 1862 (9), 183359.