A Rapid and Target-Specific Exosome Isolation Method Using Aptamer-Based Microfluidics
Selective exosome isolation is essential for advancing EV-based diagnostics, yet traditional approaches often remain slow, labor-intensive, and limited in molecular specificity. This case study (based on Zhou, Z. et al., Biosensors, 2022) presents an aptamer-based microfluidic exosome isolation method for rapid, target-specific exosome capture.
Using functionalized microchannels, high-purity isolation was achieved in minutes, demonstrating a promising approach for advanced EV analysis. This method was demonstrated at SIAT in China using Fluigent’s pressure-based flow controllers.
A paper from Shenzhen Institutes of Advances Technology
Paper: Zhou, Z.; Chen, Y.; Qian, X. Target-Specific Exosome Isolation through Aptamer-Based Microfluidics. Biosensors 2022, 12 (4), 257. https://doi.org/10.3390/bios12040257.
This study was conducted at the Shenzhen Institutes of Advanced Technology (SIAT), Chinese Academy of Sciences. SIAT is recognized for its multidisciplinary innovations in microengineering, materials science, and biomedicine. The institute integrates fundamental research, applied development, and technology translation, serving as a hub for advancing scientific knowledge and interdisciplinary collaboration.
Overview of Extracellular Vesicles Isolation and Analysis
The Biological Importance of Exosomes
Exosomes are nano-sized extracellular vesicles (typically 30-150 nm) released by nearly all cell types (Figure 1). They transport proteins, lipids, and nucleic acids, playing essential roles in intercellular communication. Their molecular content reflects the physiological or pathological state of the parent cells, which makes them useful as biomarkers for cancer, inflammation, or neurodegenerative disorders (Figure 1-2).1–4


Challenges and Limitations of Current Exosome Isolation Methods
Due to their small size and the coexistence of multiple extracellular vesicle types of EVs, the isolation of exosomes is challenging. Traditional exosome isolation methods like ultracentrifugation, size-exclusion chromatography, or precipitation often result in low purity, have long processing times and limited specificity for subpopulations.5,6
Microfluidics, combined with engineered surface chemistry, is an emerging exosome isolation technique capable of improving speed and selectivity.
Aptamers as Precision Molecular Recognition Tools for Exosome Isolation Methods
Aptamers are short DNA or RNA oligonucleotides that fold into complex 3D structures, enabling selective binding to molecular targets such as proteins, peptides, or specific EV subpopulations (Figure 3).7

Figure 3. Schematic diagram of aptamer recognition of targets to form an aptamer-target complex.8
Compared to antibodies, aptamers offer several advantages such as chemical and thermal stability, high molecular specificity, and ease of surface functionalization (Figure 4).

Figure 4. Advantages of aptamers over antibodies in clinical applicability and industrialization.8
These features make aptamers ideal candidates for integration into systems designed for advanced exosome isolation.
Aim of the Study
The goal of the study was to develop a target-specific microfluidic platform for rapid, high-purity exosome isolation using aptamer-functionalized microchannels capable of:
- Selectively capturing exosomes expressing the surface markers CD63 and PTK7, which serve as reliable identifiers of specific exosome subpopulations
- Enhancing EV isolation specificity, purity, and throughput, while demonstrating aptamer-based microfluidics as a promising platform for future diagnostics such as liquid biopsy and disease-specific EV profiling.
Methodology: Aptamer-Functionalized Microfluidic Exosome Isolation Method using Stable Flow Control
Aptamer-Based Capture Strategy
The aptamer-based capture strategy was designed as an advanced isolating method for exosomes, integrating molecular recognition with controlled microfluidic flow.
The microfluidic chip uses a surface coated with streptavidin and desthiobiotin-aptamers to selectively bind exosomes carrying CD63 or PTK7 markers. As the sample flows through the channel, these aptamers capture the target vesicles while allowing non-specific particles to pass.
A two-zone chip layout supports this process by using a micropillar zone that filters out larger debris and a capture channel that maximizes contact between the flowing sample and the aptamer surface (Figure 5). This design provides cleaner and more selective isolation of exosomes under controlled flow conditions.

Figure 5. Aptamer-based exosomes isolation microfluidics. (A) Immobilization of aptamer onto glass surface for EVs capture. (B) Prototype of PDMS/glass chip. (C) Scanning electron microscope (SEM) image of micropillars inlet. The scale bar is 500 μm.
Exosome Isolation Method using a Pressure-Driven Flow Controller
A lung cancer cell culture supernatant was processed through the microfluidic chip using a nitrogen-driven, pressure-controlled flow with Fluigent MFCSTM (Figure 6). The flow rate and residence time (approximately 10 min) were precisely regulated, ensuring reproducible exosome capture under low-shear conditions. After incubation, captured vesicles were eluted in PBS using the same stable pressure source.
For comparison, an identical sample was purified using a commercial isolation kit, providing a benchmark for yield and quality.

Figure 6. Experiment setup for exosome isolation
Results: Faster Isolation of Exosomes with Enhanced Specificity and Structural Integrity
Morphology of the isolated exosomes
Transmission electron microscopy (TEM) and atomic force microscopy (AFM) confirmed that the isolated exosomes maintained the expected cup-shaped morphology. Vesicles appeared intact, with sizes in the typical 100-200 nm range (Figure 7). Both imaging methods confirmed that pressure-based microfluidics preserve vesicle structure during capture and elution.
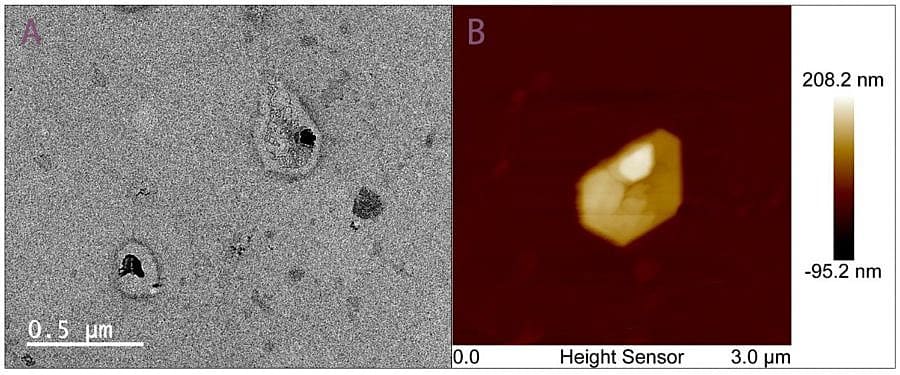
Figure 7. TEM and AFM imaging of exosomes isolated by aptamer-based microfluidics.
Size distribution and yield
Nanoparticle Tracking Analysis (NTA) was performed and compared with the commercial kit. The NTA results are illustrated in Figure 8. Across different cell culture passages, the chip and kit produced similar overall particle concentrations. The chip-isolated vesicles exhibited a smaller mean diameter, closer to the expected 30-100 nm exosome range and a narrower and more uniform size distribution.
The aptamer-based platform achieved a capture efficiency of 10⁷-10⁸ particles/mL, slightly lower than the commercial kit, but the microfluidic method produced a purer and more size-specific exosome population.

Figure 8. NTA of exosomes by using aptamer-based microfluidics and commercial kit. (A) Averaged concentration/size distribution of exosomes collected from cell culture supernatant (passage-6) by using commercial kit. (B) Averaged concentration/size distribution of exosomes collected from cell culture supernatant (passage-6) by using our device.
Exosomal marker expression (CD63 and PTK7)
Enzyme-Linked Immunosorbent Assay (ELISA) measurements of CD63 and PTK7 markers showed higher protein levels in chip-isolated samples compared to kit controls. This indicates:
- More selective capture of true exosomes
- Better enrichment of vesicles carrying the target markers
- Reduced contamination from larger and non-specific EVs
Conclusion
Aptamer-based microfluidics delivers a fast, selective, and high-purity exosome isolation method, offering a strong alternative to traditional EV separation techniques. The study from the Shenzhen Institutes of Advanced Technology demonstrates that aptamer-functionalized microchannels, combined with Fluigent’s stable pressure-based flow control, enable efficient capture of specific exosome subtypes with excellent structural integrity.
Moreover, the entire workflow requires only about 20 minutes, far faster than the hours needed for ultracentrifugation, while maintaining high marker richness and minimal processing bias.
This rapid and robust isolation strategy is fully compatible with clinical and point-of-care use, supporting applications in liquid biopsy, cancer detection, and advanced biomarker analysis. This work highlights the growing role of pressure-driven microfluidics in enabling reliable and clinically ready exosome isolation workflows.
Discover our range of flow control instruments
Expertises & Resources
-
Microfluidic Application Notes Precision Microfluidics for Magnetic Nanoparticle Encapsulation Read more
-
Microfluidics Case Studies Microfluidics-Interfaced Capillary Electrophoresis for Continuous Analysis of Nanoparticle–Bioentity Interactions Read more
-
Expert Reviews: Basics of Microfluidics Microfluidics in Drug Delivery: A New Era of Precision Medicine Read more
-
Expert Reviews: Basics of Microfluidics 10 Tips for Reliable Droplet Generation Read more
-
Expert Reviews: Basics of Microfluidics Giant Unilamellar Vesicles (GUVs) Production using Microfluidics Read more
-
Microfluidics Article Reviews Solid lipid nanoparticles for biologics and drug encapsulation Read more
-
Microfluidics White Papers Double emulsion for the generation of microcapsules – a Review Read more
References
(1) Lai, J. J.; Chau, Z. L.; Chen, S.-Y.; Hill, J. J.; Korpany, K. V.; Liang, N.-W.; Lin, L.-H.; Lin, Y.-H.; Liu, J. K.; Liu, Y.-C.; Lunde, R.; Shen, W.-T. Exosome Processing and Characterization Approaches for Research and Technology Development. Adv. Sci. 2022, 9 (15), 2103222. https://doi.org/10.1002/advs.202103222.
(2) Li, X.; Corbett, A. L.; Taatizadeh, E.; Tasnim, N.; Little, J. P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I. T. S. Challenges and Opportunities in Exosome Research—Perspectives from Biology, Engineering, and Cancer Therapy. APL Bioeng. 2019, 3 (1), 011503. https://doi.org/10.1063/1.5087122.
(3) Tzng, E.; Bayardo, N.; Yang, P. C. Current Challenges Surrounding Exosome Treatments. Extracell. Vesicle 2023, 2, 100023. https://doi.org/10.1016/j.vesic.2023.100023.
(4) Chen, J.; Li, P.; Zhang, T.; Xu, Z.; Huang, X.; Wang, R.; Du, L. Review on Strategies and Technologies for Exosome Isolation and Purification. Front. Bioeng. Biotechnol. 2022, 9. https://doi.org/10.3389/fbioe.2021.811971.
(5) He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8 (1), 237–255. https://doi.org/10.7150/thno.21945.
(6) Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomedicine 2020, 15, 6917–6934. https://doi.org/10.2147/IJN.S264498.
(7) Wolter, O.; Mayer, G. Aptamers as Valuable Molecular Tools in Neurosciences. J. Neurosci. 2017, 37 (10), 2517–2523. https://doi.org/10.1523/JNEUROSCI.1969-16.2017.
(8) Sun, H.; Zu, Y. A Highlight of Recent Advances in Aptamer Technology and Its Application. Molecules 2015, 20 (7), 11959–11980. https://doi.org/10.3390/molecules200711959. v



