Micropipette Aspiration of Red Blood Cells Mechanosensitivity and Mechanics
This case study links mechanosensitive Ca²⁺ channel activation in red blood cells to their mechanical properties. Using micropipette aspiration with a Flow EZ pressure controller, brightfield imaging measured stiffness, tension, and viscosity, while fluorescence detected Ca²⁺ transients. Activation pressure shifted before mechanical changes became significant, offering a precise method to study mechanosensitivity–mechanical correlations in single cells.
A Paper From the National Research Council of Italy
Braidotti, N.; Ciubotaru, C. D.; Rizzo, D.; Bergamo, L.; Bernareggi, A.; Cojoc, D., Investigating Mechanosensitive Channels Activation in Concert with the Mechanical Properties of Red Blood Cells. Discov Mechanical Engineering 2023, 2 (1), 18. https://doi.org/10.1007/s44245-023-00026-3.
This study results from a collaboration between the University of Trieste’s Departments of Physics and Life Sciences and the Istituto Officina dei Materiali (IOM) of the National Research Council of Italy (CNR). IOM conducts interdisciplinary research in condensed matter physics, nanoscience, and biophysics, focusing on nanoscale knowledge to develop advanced materials, biosystems, and devices. Its work integrates theory, synthesis, characterization, and nanofabrication, addressing national and European priorities in areas such as Health and Nanomedicine, Quantum Technologies, Climate and Energy, and Digital Industry.
Introduction to Red Blood Cell Mechanics
Red blood cells (RBCs) transport oxygen to tissues and remove carbon dioxide, a task that requires deformability to navigate the narrow vessels of the circulatory system. This flexibility is sustained throughout their 120-day lifespan, aided by their biconcave shape, elastic membrane, and a cytoskeletal network of actin and spectrin proteins that resist bending and shear stress.1–5
Pathological conditions such as sickle cell disease, malaria, diabetes, and sepsis can disrupt these mechanical properties, impairing blood flow. To study such changes, techniques like micropipette aspiration, microfluidics, atomic force microscopy, and optical tweezers apply controlled forces to individual cells, allowing measurement of parameters such as Young’s modulus, bending modulus, membrane tension, and viscosity. Surface area and volume further aid in assessing RBC health and functionality.6
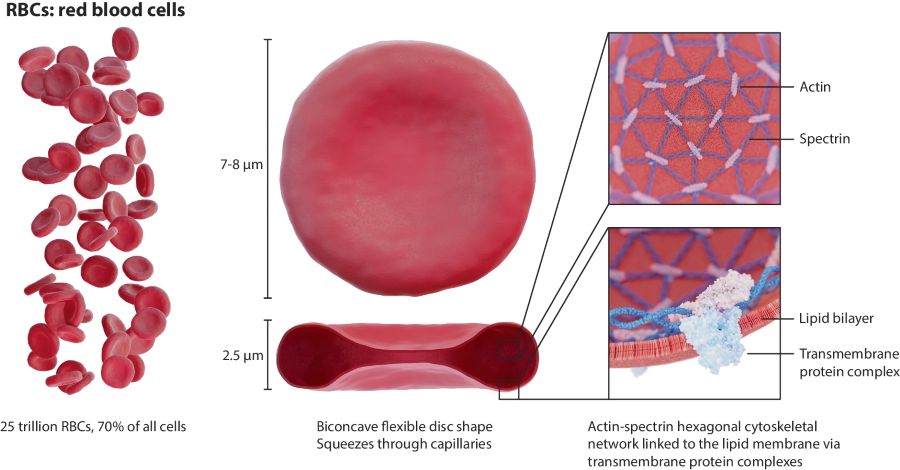
Figure 1: RBC characteristics (from Waeterschoot, J. et al. Nat Commun 2024, 15 (1), 2504).
Ion Channel Regulation in Red Blood Cells
Red blood cell (RBC) volume is tightly regulated by the flow of Calcium (Ca²⁺), Sodium (Na⁺), and Potassium (K⁺) through membrane channels. A key channel is Piezo1, a large mechanosensitive protein that opens in response to membrane tension, allowing Ca²⁺ influx. This calcium entry activates the Gárdos channel, which causes K⁺ and water to exit the cell, reducing volume. Mutations in Piezo1 are linked to RBC dehydration disorders.3,7
Mechanosensitive channel function depends on external forces and the cell’s mechanical properties. These factors are often studied separately. New techniques combining fluorescence and brightfield imaging, micropipette aspiration of RBC’s with calcium monitoring, allow for the simultaneous analysis of mechanical stress and channel activity in individual RBCs, improving understanding of their interaction.8–11
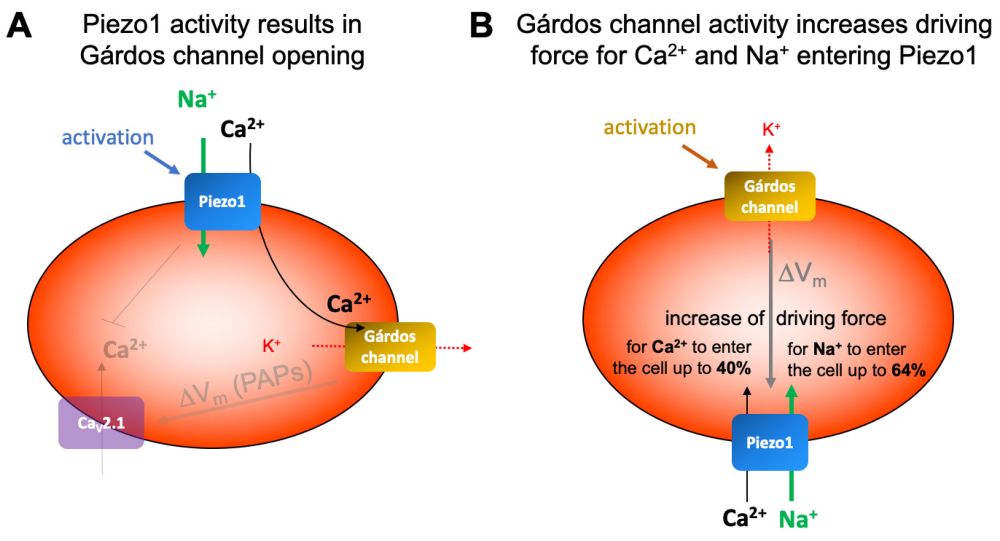
Figure 2: Schematic presentation of the interplay of Gárdos channel and Piezo1 (from Petkova-Kirova, P. et al. IJMS 2024, 25 (3), 1416).
Aim of the Study
In this study, a bimodal imaging technique combined with micropipette aspiration was utilized to simultaneously monitor calcium channel activation and mechanical deformation in individual human RBCs. Fluorescence Ca²⁺ imaging was employed to determine the activation pressure of mechanosensitive channels, while mechanical properties such as Young’s modulus, membrane tension, and viscosity were quantified by measuring cell deformation in response to controlled aspiration pressures. Validation of the method was performed by analyzing RBCs at three-time intervals (0–20, 20–40, and 40–60 minutes) after sample preparation, revealing dynamic changes in both channel activation thresholds and mechanical behavior.
Webinar Replay
RBC Mechanics and Channel Activation by Micropipette Aspiration
Role of Piezo1 Channel Activity in RBCs and Its Link to Alzheimer’s Disease.
Materials and Methods
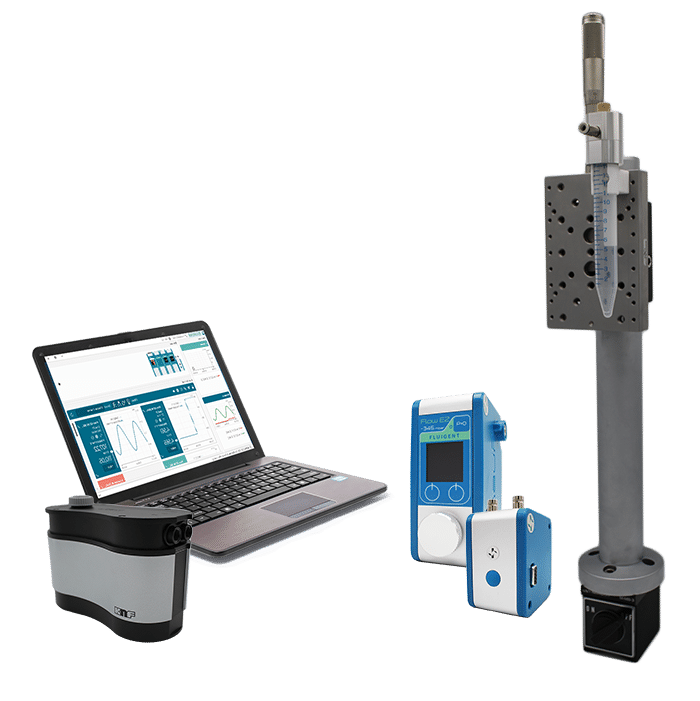
A combined micropipette aspiration and bimodal imaging system was employed to simultaneously monitor mechanical deformation and calcium signaling in single RBCs. The setup incorporated the Fluigent Flow EZ pressure controller capable of regulating negative pressures from 0 to minus 60 mbar. This enables the gradual attraction of cells to the pipette tip and their progressive deformation into the capillary. The aspiration module was mounted on an inverted microscope equipped with a motorized stage, micromanipulator, and dual-sensor camera to capture both brightfield and fluorescence images. Pressure ramps, typically applied at minus 2 mbar/s, allowed sequential observation of cell tether formation and deformation.
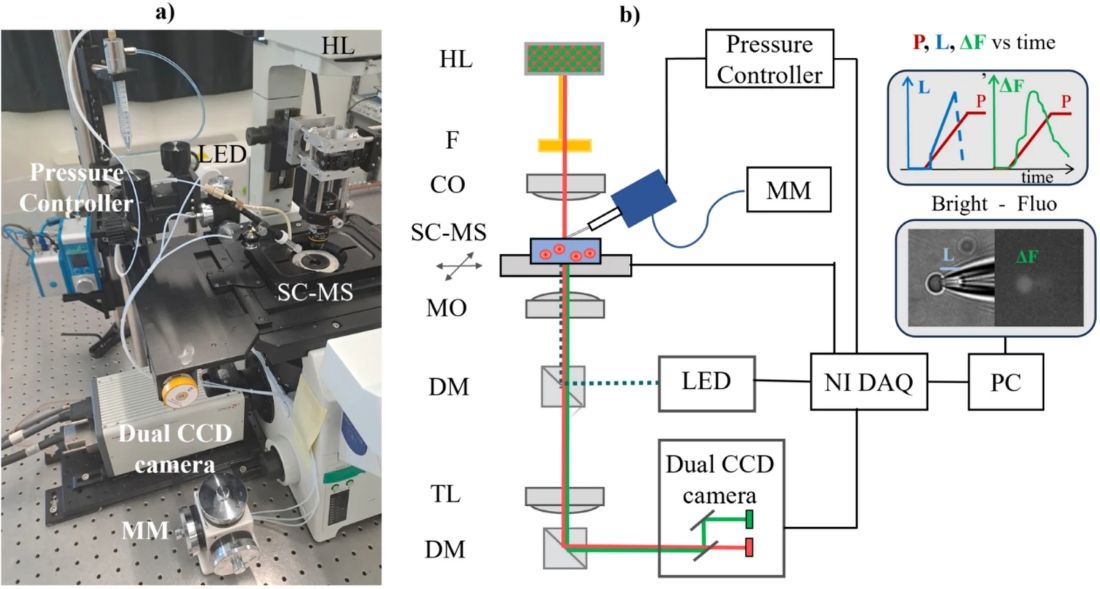
Figure 3: Combined micropipette aspiration and bimodal imaging setup (from Braidotti, N. et al. Discov Mechanical Engineering 2023, 2 (1), 18).
All components, including the pressure regulation unit, illumination source, and camera, were synchronized via a digital acquisition system with custom control software, ensuring precise temporal correlation of pressure, mechanical deformation, and fluorescence data.
Human RBCs were isolated from anonymized donor blood, stored at 4 °C, and used within three days. Following standard washing procedures, cells were incubated with a calcium-sensitive fluorescent dye, rinsed, and resuspended in physiological buffer for experiments. Samples were analyzed within one hour of preparation to evaluate temporal changes in mechanical and calcium signaling properties.
Example of cell rupture inside the capillary as a consequence of high pressure levels (from Braidotti, N. et al. Discov Mechanical Engineering 2023, 2 (1), 18)
Example of accelerated recording showing the deformation of an aspirated cell and relative fluorescent signalindicating Ca influx (from Braidotti, N. et al. Discov Mechanical Engineering 2023, 2 (1), 18)
Key Mechanical Parameters and Activation Pressures in Micropipette Aspiration of RBCs
This table summarizes the main pressures and mechanical properties used to study RBC behavior during micropipette aspiration, based on pressure and fluorescence data.
| Parameter | Description | Formula / Definition |
|---|---|---|
| Fluorescence Signal (ΔF) | Normalized cell fluorescence corrected by background | ΔFs = (Fs-F0) /F0 ΔF= ΔFs – ΔFb where ΔFs: mean fluorescence in cell region of interest F0: baseline mean fluorescence; ΔFb: background fluorescence |
| Contact Pressure (Pc) | Pressure at first fluorescence elevation | Initial pressure when cell touches pipette |
| Activation Pressure (Pa) | Pressure at second fast fluorescence elevation, triggering Ca²⁺ influx via mechanosensitive channels | Pressure where fluorescence reaches ΔF=ΔFc+c×(ΔFm−ΔFc) with c=0.1 |
| Maximum Aspiration Pressure (Pma) | Pressure when fluorescence drops to 90% max (cell detachment) | ΔFma=0.9×ΔFm |
| Young’s Modulus (E) | Cell elasticity (Pa) | E=11.4×(ΔP/ΔL)×Dp |
| Cortical Tension (Tc) | Membrane tension (N/m) | Tc=1.1×E×Dp |
| Tether Velocity (Vt) | Speed of membrane elongation (µm/s) | Vt=ΔL/Δt |
| Cell Viscosity (ν) | Viscous resistance (Pa·s) | ν= (Dp ΔP) / (12(ΔL/Δt)(1-Dp/Dc)) |
With :
- ΔP= Pma−Pc
- ΔL= L−L0
- Dp = pipette diameter
- Dc = cell diameter
- L0, Lma = tether lengths at contact and max elongation
- Δt = elongation time
Proof of Concept: Correlation Between Activation Pressure and Mechanical Properties in Human RBCs
The relationship between the pressure required to activate mechanosensitive channels and the mechanical properties of human RBCs was investigated using micropipette aspiration of red blood cells. Cells were grouped by time in the sample chamber: P1 (0–20 min), P2 (20–40 min), and P3 (40–60 min). A typical experiment is illustrated in Figure 4: panel (a) shows the pressure values Pc, Pa, and Pma derived from the differential fluorescence ΔF curve, and panel (b) displays bimodal images of cell morphology and fluorescence at two instants—cell contact and tether formation in the micropipette—indicating channel activation.
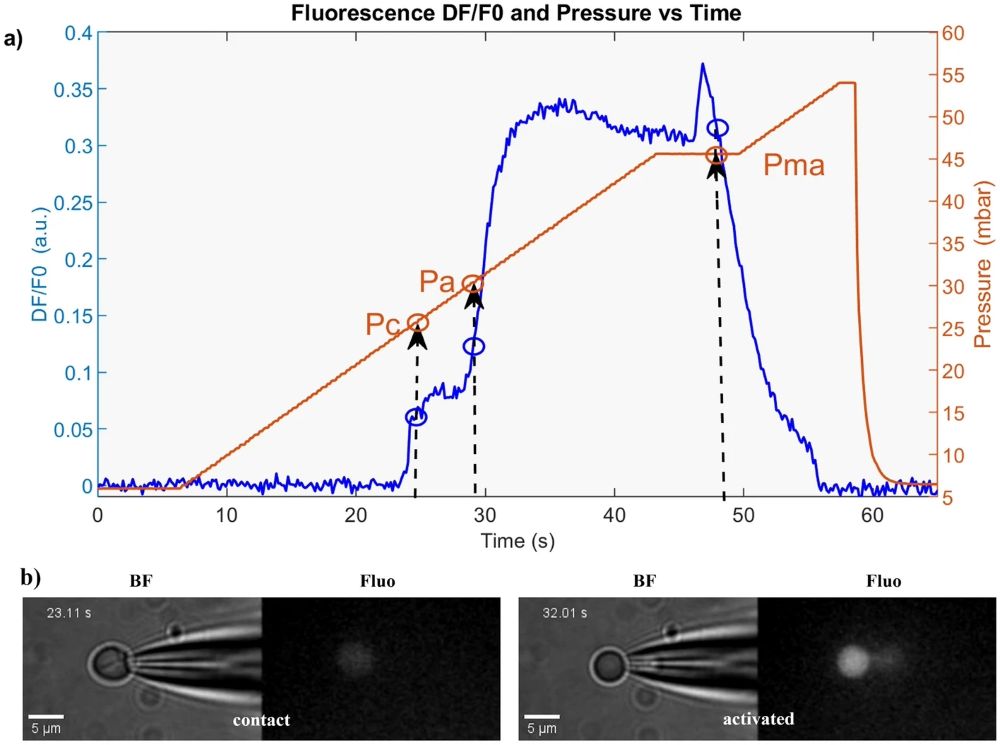
Figure 4: Example from micropipette aspiration of red blood cells: a Pc, Pa, Pma from ΔF curve. b Images show cell contact (left) and tether with increased fluorescence (right) (from Braidotti, N. et al. Discov Mechanical Engineering 2023, 2 (1), 18).
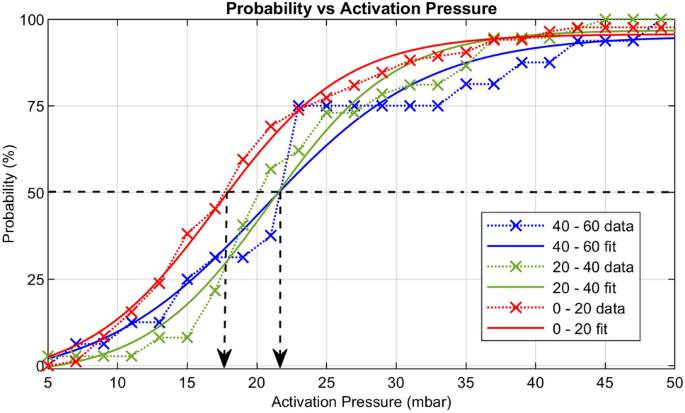
Figure 5: Activation Pressure distributions: P1 (red), P2(green) and P3 (blue) (from Braidotti, N. et al. Discov Mechanical Engineering 2023, 2 (1), 18).
The probability of channel activation as a function of pressure was fitted with a Boltzmann function (Figure 5), revealing lower P50 values for P1 compared to P2 and P3. Mean activation pressures increased with storage time, with a significant difference between P1 and P2, indicating a rapid decline in mechanosensitivity.
Mechanical parameters including Young’s modulus (E), cortical membrane tension (Tc), tether flow velocity (Vt), and viscosity (ν) were measured. Table 1 shows that E, Tc, and ν increased over time, while Vt decreased, reflecting short-term stiffening, higher viscosity, and increased cortical tension.
| Parameter | P1 (0–20 min) Mean ± SD | P2 (20–40 min) Mean ± SD | P3 (40–60 min) Mean ± SD |
|---|---|---|---|
| Activation pressure, Pa [mbar] | 22.55 ± 11.3 | 24.76 ± 8.8 | 25.47 ± 11.5 |
| Young’s modulus, E [Pa] | 57.83 ± 46.3 | 67.24 ± 49.8 | 113.57 ± 48.4 |
| Membrane tension, Tc [pN/µm] | 96.7 ± 76.1 | 125.1 ± 87.5 | 187.4 ± 79.8 |
| Tether velocity, Vt [µm/s] | 1.13 ± 1.0 | 1.12 ± 0.9 | 0.54 ± 0.5 |
| Viscosity, ν [Pa·s] | 208 ± 204 | 277 ± 244 | 527.02 ± 331.5 |
Box plots and Wilcoxon tests (Figure 5) confirmed significant differences between P3 and the earlier pools, while P1 and P2 were similar. Changes in channel activation preceded measurable alterations in mechanical properties, suggesting early modulation by local membrane components, followed by cytoskeletal reorganization. These findings indicate that even short storage times in the chamber can substantially affect both RBC mechanosensitivity and mechanical behavior.
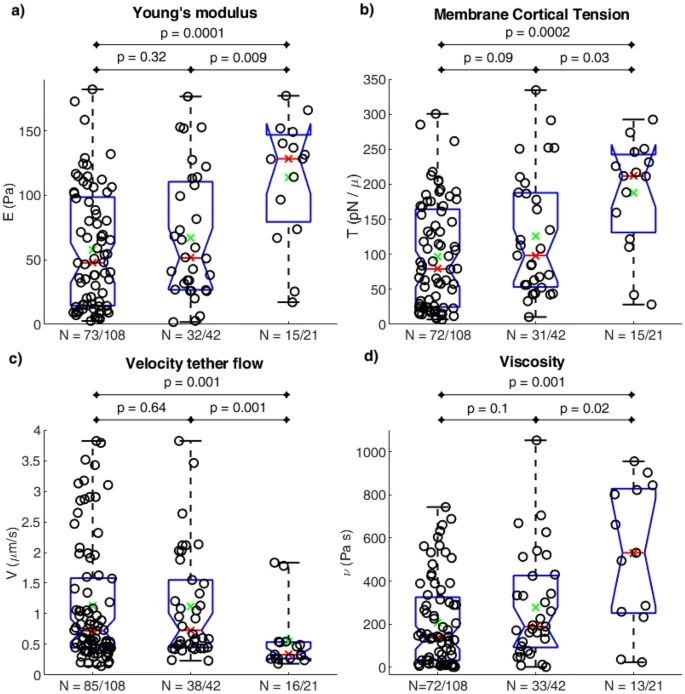
Figure 6: Box plots with Wilcoxon test p-values for mechanical parameters: Young’s modulus (a), cortical membrane tension (b), tether flow velocity (c), and viscosity (d). N indicates the number of cells (and responding cells) within the practical ranges (from Braidotti, N. et al. Discov Mechanical Engineering 2023, 2 (1), 18).
Conclusion
This study introduces a bimodal microscopy method using the Fluigent Flow EZ pressure controller for precise micropipette aspiration. This allows for the simultaneous measurement of mechanosensitive channel activation pressure and mechanical properties of red blood cells. Brightfield and fluorescence imaging tracked cell deformation and channel activity concurrently. Activation pressure changed earlier and stabilized before mechanical properties altered during one hour of storage.
These findings validated the method and were applied in research linking increased Piezo1 activity in RBCs to Alzheimer’s disease-related dementia, highlighting the clinical significance of mechanosensitive channels.12
Related Products
Related Expertises
-
Expert Reviews: Basics of Microfluidics 10 Tips for Reliable Droplet Generation Read more
-
Microfluidics Case Studies Controlled Flow System for Altering Cardiac Mechanical Load in Zebrafish Model Read more
-
Microfluidics White Papers An exploration of Microfluidic technology and fluid handling Read more
-
Expert Reviews: Basics of Microfluidics Micropipette aspiration of cells and tissues Read more
References
1. Braidotti, N. et al. Investigating mechanosensitive channels activation in concert with the mechanical properties of red blood cells. Discov. Mech. Eng. 2, 18 (2023).
2. Besedina, N. A. et al. Persistent red blood cells retain their ability to move in microcapillaries under high levels of oxidative stress. Commun. Biol. 5, 659 (2022).
3. Cahalan, S. M. et al. Piezo1 links mechanical forces to red blood cell volume. eLife 4, e07370 (2015).
4. Gokhin, D. S. et al. Dynamic actin filaments control the mechanical behavior of the human red blood cell membrane. Mol. Biol. Cell 26, 1699–1710 (2015).
5. Li, X., Peng, Z., Lei, H., Dao, M. & Karniadakis, G. E. Probing red blood cell mechanics, rheology and dynamics with a two-component multi-scale model. Philos. Transact. A Math. Phys. Eng. Sci. 372, 20130389 (2014).
6. Peng, Z. et al. Lipid bilayer and cytoskeletal interactions in a red blood cell. Proc. Natl. Acad. Sci. U. S. A. 110, 13356–13361 (2013).
7. Syeda, R. et al. Piezo1 Channels Are Inherently Mechanosensitive. Cell Rep. 17, 1739–1746 (2016).
8. Mohi, S. M., Saadon, H. L. & Khalaf, A. A. Laser tweezers as a biophotonic tool to investigate the efficacy of living sickle red blood cells in response to optical deformation. Biophys. Rev. 13, 173–184 (2021).
9. Guck, J. et al. The Optical Stretcher: A Novel Laser Tool to Micromanipulate Cells. Biophys. J. 81, 767–784 (2001).
10. Barns, S. et al. Investigation of red blood cell mechanical properties using AFM indentation and coarse-grained particle method. Biomed. Eng. OnLine 16, 140 (2017).
11. Lee, M. Calculating the Difference in Stiffness of Living T Cells Through Micropipette Aspiration. McKelvey Sch. Eng. Theses Diss. (2023).
12. Sitnikova, V. et al. Increased activity of Piezo1 channel in red blood cells is associated with Alzheimer’s disease‐related dementia. Alzheimers Dement. 21, (2025).