Microfluidics-Interfaced Capillary Electrophoresis for Continuous Analysis of Nanoparticle–Bioentity Interactions
This case study highlights a modular microfluidic capillary electrophoresis platform with LED-based fluorescence detection for continuous monitoring of nanoparticle–bioentity interactions. The plug-and-play design uses off-the-shelf components, with Push-Pull pressure and vacuum controllers enabling precise, pulseless flow to mimic biofluid circulation. The platform was applied to track functionalized magnetic nanoparticles and their interaction with dopamine, demonstrating its potential for nanomedicine and drug delivery research.
A Paper from the BioMEMS and Nanoscale Engineering Group
Paper: Zafar, J.; Smadja, C.; Fabre, J.; Taverna, M.; Secret, E.; Siaugue, J.-M.; Mai, T. D. Sensors and Actuators B: Chemical 2025, 439, 137841
This study results from a collaboration between Institut Galien Paris-Saclay (CNRS, Université Paris-Saclay), Adelis SAS, and PHENIX (CNRS, Sorbonne Université). At the Institut Galien Paris-Saclay, the Protéines et Nanotechnologies en Sciences Analytiques (PNAS) team specializes in the development of miniaturized analytical techniques for studying biomolecules such as proteins and peptides. Their work focuses on advanced methods for biomolecular separation, quantification, and interaction analysis, including the integration of electrokinetic techniques and mass spectrometry, as well as the development of biosensors for healthcare applications.


How Can Microfluidics Interfaced With Capillary Electrophoresis Advance Nanoparticle Interaction Studies?
Nanoparticles have emerged as key target analyte carriers in biomedical applications, particularly in drug delivery, diagnostics, and imaging. Understanding their interactions with biological entities, such as proteins, cells, and biofluids, is critical for optimizing their effectiveness in these applications. However, studying these interactions in real-time, within dynamic biological environments, presents a significant challenge due to the complex and ever-changing nature of biofluids.1–3
Capillary electrophoresis has proven to be a powerful tool for the separation and characterization of biomolecules and nanoparticles due to its high sensitivity and precision. However, conventional capillary electrophoresis systems, often designed for bench-top analysis, are not well suited for continuous, on-site sampling or real-time monitoring of nanoparticle-bioentity interactions.4–6
Microfluidics-interfaced capillary electrophoresis offers a promising solution to overcome these limitations. By integrating off-the shelf microfluidic systems with capillary electrophoresis, it is possible to create a highly adaptable platform that allows for continuous flow, precise sample handling, and real-time analysis. This integration can not only facilitate better control over experimental conditions but also improves the overall efficiency and sensitivity of nanoparticle-bioentity interaction studies.1,7,8
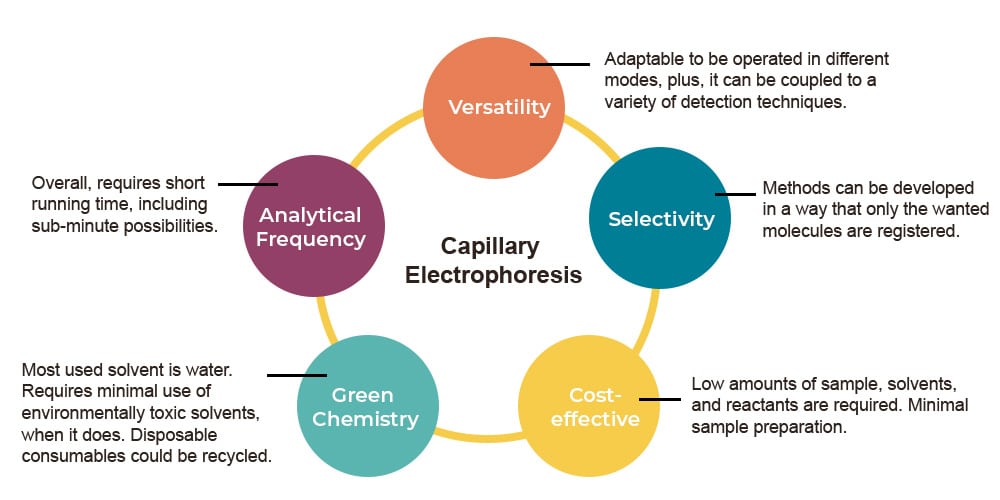
Figure 1: The advantages of capillary electrophoresis as an analytical tool (from Moreira, O. et al.; Braz. J. Anal. Chem. 2022).
Aim of the Study
This study aims to design a modular microfluidics-interfaced capillary electrophoresis platform for continuous monitoring of interactions between functionalized nanoparticles and biologically relevant molecules under dynamic flow conditions. The system integrates microfluidic modules for pulseless flow and automated injection with LED-based fluorescence detection for real-time, sensitive analysis. As aproof of concept, the platform characterizes the interaction between silica-coated magnetic nanoparticles and dopamine in artificial cerebrospinal fluid. This interaction serves as an example of how nanoparticles can be used in targeted drug delivery systems, where they transport dopamine and enable its controlled release in the central nervous system.
This approach can demonstrate the capability of microfluidics-interfaced capillary electrophoresis to enhance the understanding of nanoparticle behavior in biofluid-like environments.
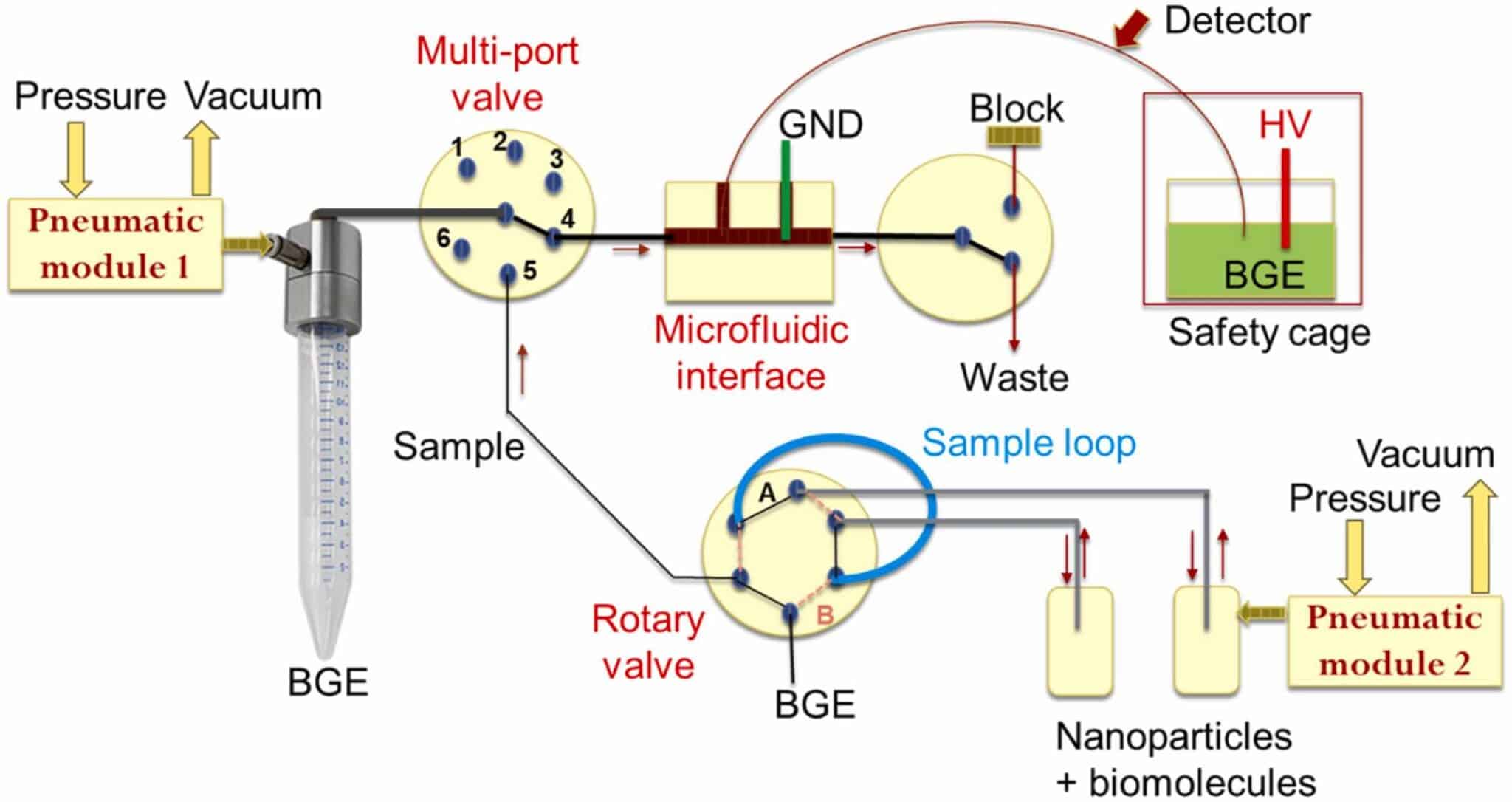
Figure 2: Schematic drawing of the microfluidics-interfaced capillary electrophoresis setup (GND: ground electrode; HV: High voltage electrode; BGE: background electrolyte).1
Methodology toA Capillary Electrophoresis System Coupled with Microfluidics
The microfluidics-interfaced capillary electrophoresis platform was designed using modular components to enable precise, stable, and continuous fluid handling for automated sampling and injection. The microfluidic system was assembled from Fluigent modules, including Push-Pull pressure controllers for pulseless bidirectional flow, M-Switch and L-Switch valves for dynamic channel selection, and 2-Switch for flexible fluid routing. The pressure and vacuum required for flow control were provided by compressed air (using Fluigent FLPG) and a vacuum source. All microfluidic modules were controlled, and experimental steps were managed using OxyGEN software, ensuring practicality and automated execution of fluid manipulation steps.
The electrophoresis setup consisted of polyimide-coated fused silica capillaries and a high-voltage power supply for dual-polarity operation (±30 kV). Detection was performed using a modular LED-induced fluorescence system with a 480 nm excitation source, optical focusing components, and a photomultiplier-based sensor for signal acquisition.
Silica-coated magnetic nanoparticles were synthesized by sol-gel encapsulation of maghemite cores, with fluorescent labels incorporated during shell formation. Their size and morphology were characterized by electron microscopy and dynamic light scattering.
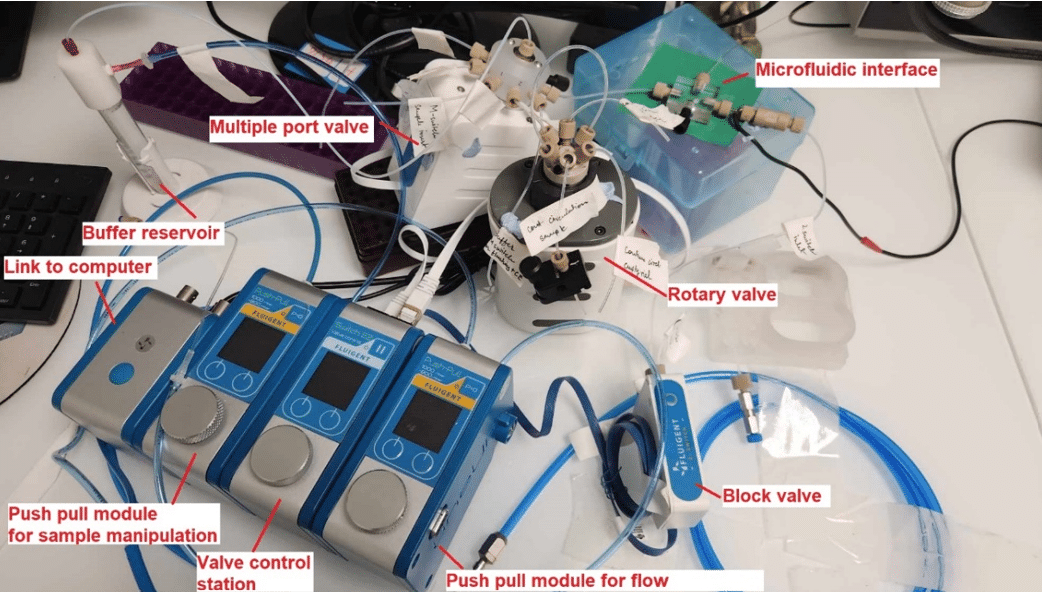
Figure 3: Photo of the microfluidics-interfaced capillary electrophoresis system modules.1
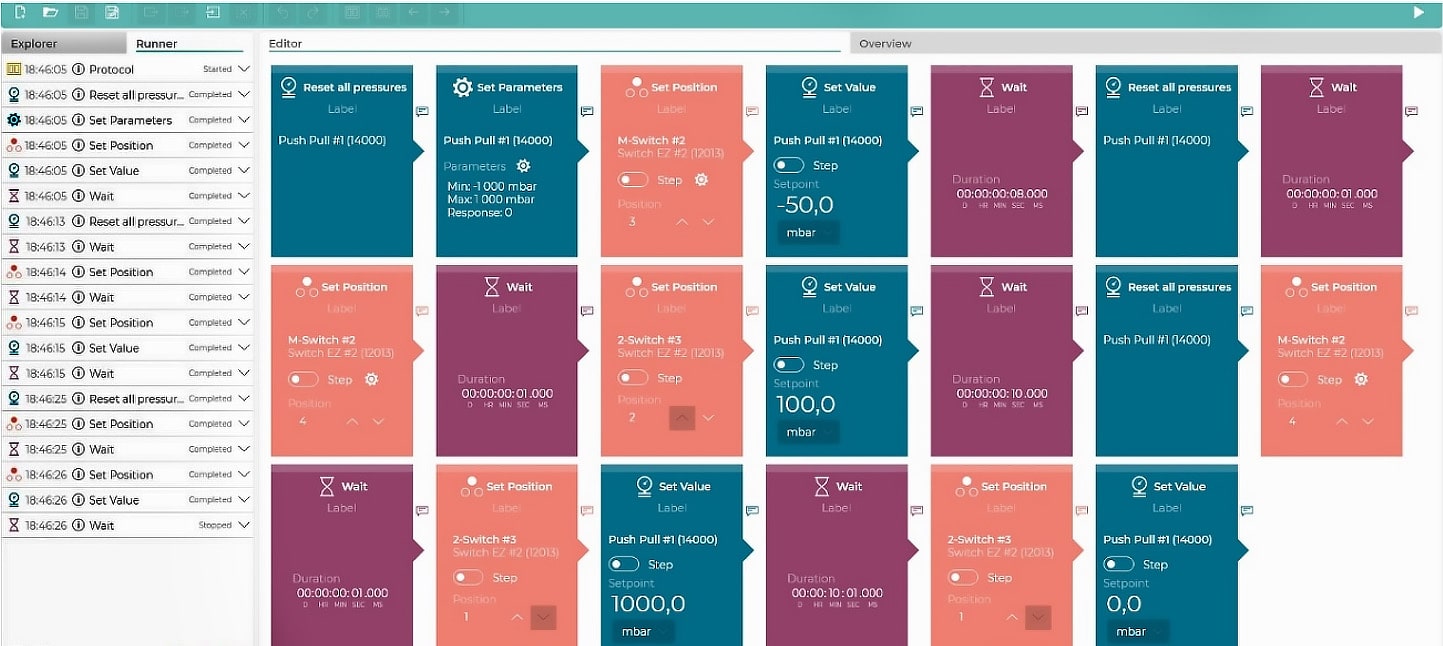
Figure 4: Protocol used in OxyGEN for fluidic steps.1
“Preliminary efforts were made to build systems with syringe pumps and different arrangements of sequential injection valves. However, with all tested setups we encountered failure to create stable and constant pressure for sample injection and manipulation of liquids in the tubings and through the capillary. It is indeed not trivial to generate a pulseless flow, as the strokes from the piston movement are inevitable. To overcome these problems, all syringe pumps and valves were finally replaced with the modular setup of pressure/vacuum controllers and valves, conventionally employed for microfluidic operations, which can be connected via ‘plug and play’ mode.”
From Zafar, J. et al.; Sensors and Actuators B: Chemical 2025, 439, 137841.
Proof of Concept: Continuous Monitoring of Nanoparticle–Dopamine Interactions
The modular microfluidic capillary electrophoresis system was developed to address the limitations of traditional capillary electrophoresis, particularly in laboratories with minimal infrastructure. It combines the advantages of both bench-top and microchip systems through a modular, plug-and-play design. The system replaces syringe pumps with Fluigent’s Push-Pull pressure and vacuum controllers, providing stable, pulseless flow. With precise positive and negative pressures for sample injection (+30 to +100 mbar) and capillary flushing (−800 to +1000 mbar), the system enables full automation of fluidic manipulation, providing stable injection pressures with relative standard deviation (RSD) below 2.5%, and reproducible delivery times (RSD below 0.8%) (Table 1).
Table 1: Performance data for the test on injection reproducibility realized with the modular microfluidic capillary electrophoresis system.1
| Injection conditions (pressure and time) | Mean value of peak area (mV·s) | RSD % (n = 4) Peak area | RSD % (n = 4) Delivery time |
|---|---|---|---|
| 50 mbar – 10 s | 2.17 | 1.03 | 0.54 |
| 50 mbar – 20 s | 4.72 | 0.85 | 0.40 |
| 50 mbar – 30 s | 5.62 | 1.77 | 0.15 |
| 50 mbar – 40 s | 6.70 | 1.71 | 0.14 |
| 100 mbar – 10 s | 3.43 | 1.25 | 0.27 |
| 100 mbar – 20 s | 4.89 | 2.47 | 0.69 |
| 100 mbar – 30 s | 6.24 | 1.40 | 0.76 |
| 100 mbar – 40 s | 7.54 | 1.25 | 0.17 |
The system also features microfluidic-interfaced fluorescence detection, providing cost-effective, high-sensitivity analysis for fluorescent analytes. It ensures high linearity and stability, making it suitable for monitoring fluorescent nanoparticles and their interactions with biomolecules.
As a proof of concept, the microfluidic-interfaced capillary electrophoresis system enabled baseline separation of sulfobetaine-functionalized magnetic nanoparticles from residual fluorophore using an optimized background electrolyte (Tris/MOPS, 50 mM, pH 9.5) (figure 5). This separation showed good repeatability, with RSD for peak areas and migration times below 4.3% and 2.9%, respectively. The ability to separate nanoparticles using electrophoresis is vital for ensuring the purity, size, and charge characteristics of drug delivery systems, which is crucial for optimizing their effectiveness in targeting specific tissues and controlling drug release.
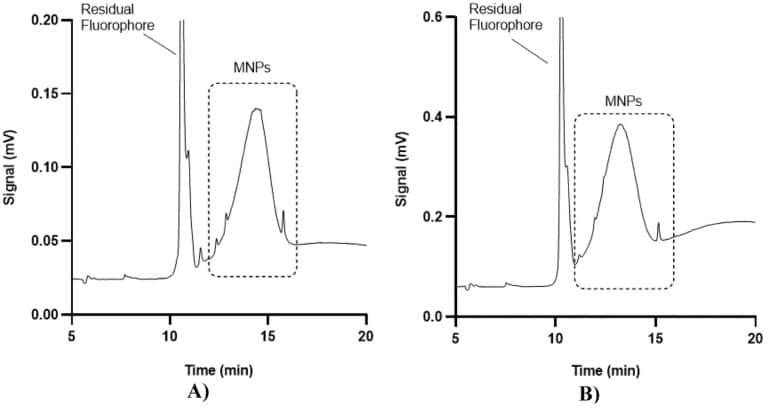
Figure 5: Electropherograms of two different categories of magnetic nanoparticles functionalized with sulfobetaine. A) 47 nm spheric; B) 125 nm non spheric.1
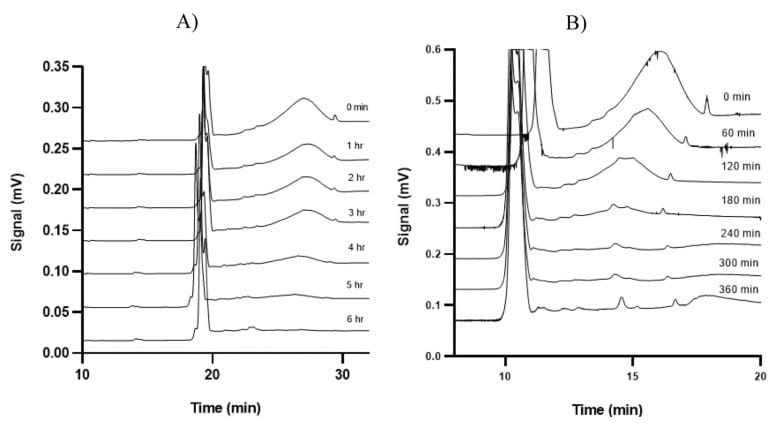
Figure 6: Time-dependent electropherograms of 60 nm of the functionalized nanoparticles interacting with dopamine in continuous microflows of (A) PBS and (B) artificial cerebrospinal fluid.1
The interaction between magnetic nanoparticles and dopamine was monitored in continuous microflow under physiological conditions. At a dopamine concentration of 20 µM, the nanoparticle signal disappeared after 6 hours, indicating complete interaction. At higher dopamine concentrations of 50 µM and 200 µM, the complete interaction occurred after 4 hours and 2 hours, respectively. The interaction kinetics were consistent in both phosphate-buffered saline (PBS) and artificial cerebrospinal fluid, demonstrating the robustness of this capillary electrophoresis system in diverse physiological conditions.
Conclusion
In summary, a microfluidics-interfaced capillary electrophoresis platform with LED-induced fluorescence was developed using off-the-shelf components. The modular design allows users to create customized analytical setups with operations not typically available in commercial systems, including automated continuous flow sampling. The system successfully separated fluorescent magnetic nanoparticles functionalized with sulfobetaine, demonstrating its potential for drug delivery and diagnostics. Additionally, it effectively monitored the interaction between nanoparticles and dopamine in continuous microflow, offering a compact and flexible alternative to traditional microfluidic capillary electrophoresis systems.
Related Solutions
Related Resources
-
Expert Reviews: Basics of Microfluidics Microfluidics in Drug Delivery: A New Era of Precision Medicine Read more
-
Expert Reviews: Basics of Microfluidics Microfluidic Flow Sensing Technologies, A Review Read more
-
Microfluidics Article Reviews Microfluidic technology for engineered nanoparticles in nanomedicine Read more
-
Microfluidic Application Notes Capillary electrophoresis using microfluidic, electrophoretic, and optic modules Read more
-
Expert Reviews: Basics of Microfluidics The Importance of Flow Control Stability in Microfluidics Read more
References
1. Zafar, J. et al. Microfluidics-interfaced capillary electrophoresis coupled with modular LED-based fluorescent detection: A new tool for continuous monitoring of the interaction between nanoparticles and bio-entities. Sens. Actuators B Chem. 439, 137841 (2025).
2. Ma, Z., Mohapatra, J., Wei, K., Liu, J. P. & Sun, S. Magnetic Nanoparticles: Synthesis, Anisotropy, and Applications. Chem. Rev. 123, 3904–3943 (2023).
3. Materón, E. M. et al. Magnetic nanoparticles in biomedical applications: A review. Appl. Surf. Sci. Adv. 6, 100163 (2021).
4. Moreira, O. et al. Capillary Electrophoresis Applied to Human Urine Analysis for Clinical Diagnosis: New Trends and Perspectives. Braz. J. Anal. Chem. (2022) doi:10.30744/brjac.2179-3425.RV-13-2022.
5. Hartung, S., Minkner, R., Olabi, M. & Wätzig, H. Performance of capillary electrophoresis instruments – State of the art and outlook. TrAC Trends Anal. Chem. 163, 117056 (2023).
6. Schöneborn, H. et al. Novel Tools towards Magnetic Guidance of Neurite Growth: (I) Guidance of Magnetic Nanoparticles into Neurite Extensions of Induced Human Neurons and In Vitro Functionalization with RAS Regulating Proteins. J. Funct. Biomater. 10, 32 (2019).
7. Nguyen, N. V. T. et al. On-line dual-stage enrichment via magneto-extraction and electrokinetic preconcentration: A new concept and instrumentation for capillary electrophoresis. Anal. Chim. Acta 1255, 341141 (2023).
8. Nguyen, N. V. T. et al. Electroosmotic flow modulation for improved electrokinetic preconcentration: Application to capillary electrophoresis of fluorescent magnetic nanoparticles. Anal. Chim. Acta 1161, 338466 (2021).






