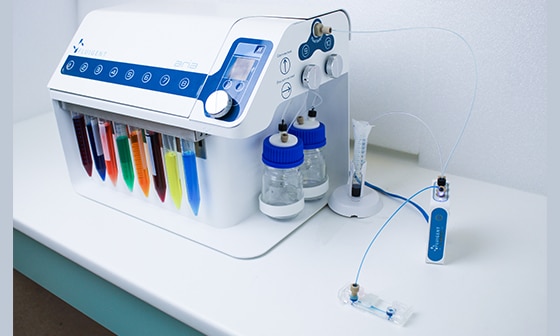
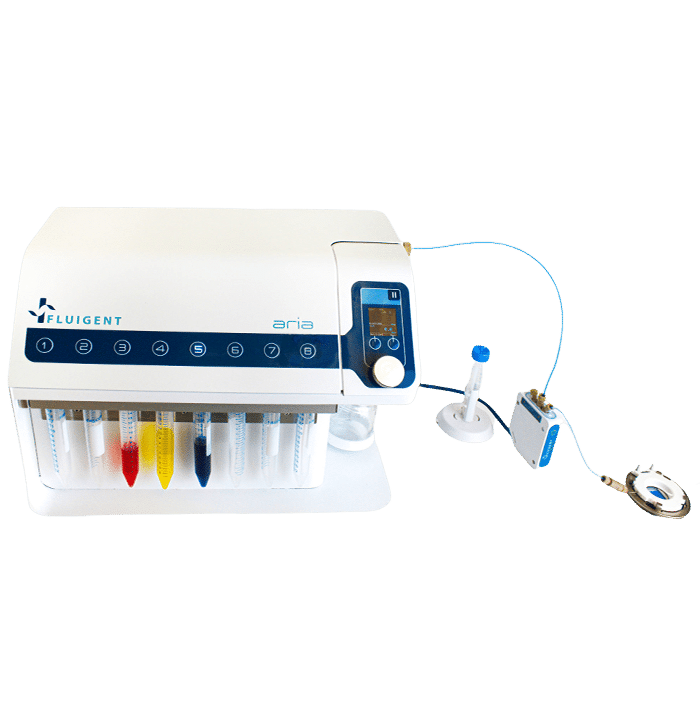
Exchange-PAINT: Automated on-stage staining with Aria
Multiplexed DNA-PAINT using Aria microfluidics and ZEISS Elyra 7: automated staining and imaging high-precision super-resolution imaging. This case study is in collaboration with the Molecular Imaging and Bionanotechnology Research Group, Max-Planck-Institute of Biochemistry and Carl Zeiss Microscopy, Germany. Explore the technical details by downloading the full application note.


What is Exchange-PAINT?
Exchange-PAINT is a multiplexed single-molecule localization microscopy (SMLM) method based on DNA-PAINT. It allows the detection of different molecular targets at 4-5 nm precision in the same sample by using orthogonal DNA docker and imager strands to image multiple targets sequentially.
DNA-PAINT (DNA points accumulation for imaging in nanoscale topography) is a super-resolution microscopy method that uses short bursts of binding between glowing DNA probes and their target to create very detailed images at the nanoscale.
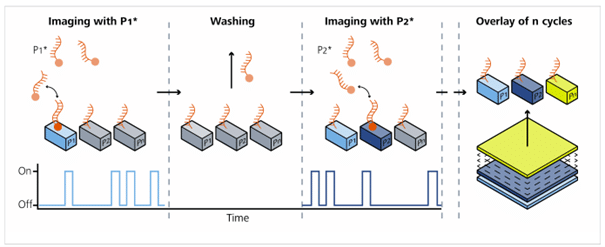
As shown in Figure 1, the sample is stained with docking strands (P1, P2, P3). Each target is tagged with a unique DNA strand that its complementary imager will bind to. For example, the P1* imager is added; it transiently binds and unbinds to the docking strand. These short binding events produce blinking fluorescence signals that are acquired by the microscope. Next, the imager solution is washed out with buffer, without chemical bleaching. Then the target P2 is visualized using the P2* docking strand, and so on. This method is performed iteratively. Finally, the collected images are computationally aligned, and fiducial markers are used to correct for drift.
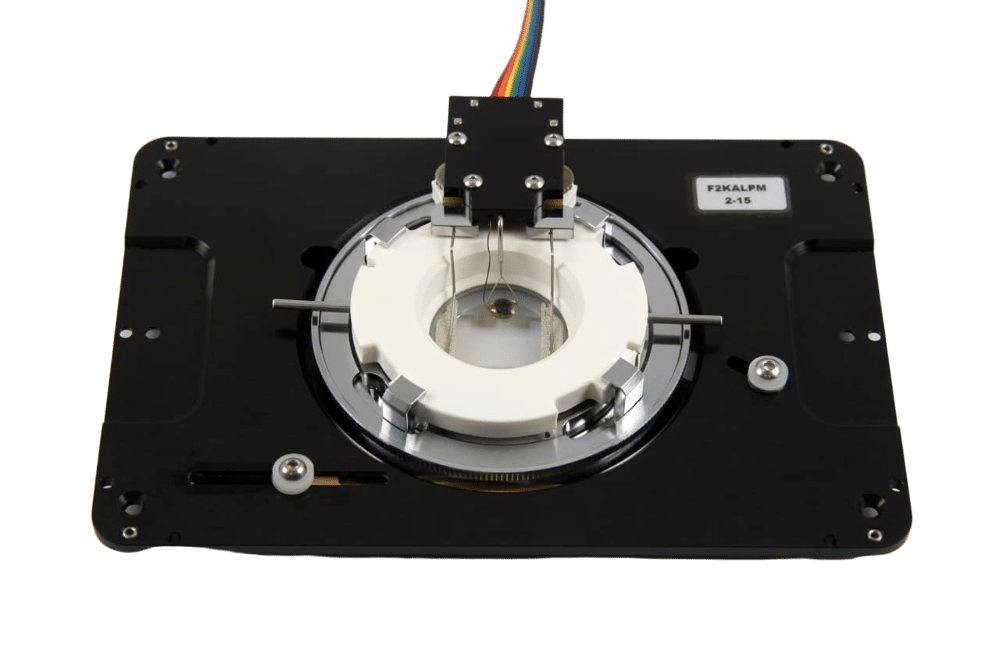
Exchange-PAINT Experimental Set Up
Automating the fluid delivery on-stage with Fluigent’s Aria keeps the field of view aligned between cycles and automates the assay. By removing tedious manual pipetting steps, errors are reduced. It enables high-precision multiplexed super-resolution imaging with ZEISS Elyra 7.
- On-stage staining and automated cyclical imagers delivery prevent loss of imaging positions between rounds.
- Direct software integration (Aria ↔ ZEN) allows synchronized fluid handling and image acquisition.
Final localization precision reported at ~4–5 nm for origami and cell experiments, with resulting resolutions down to ~9–12 nm.
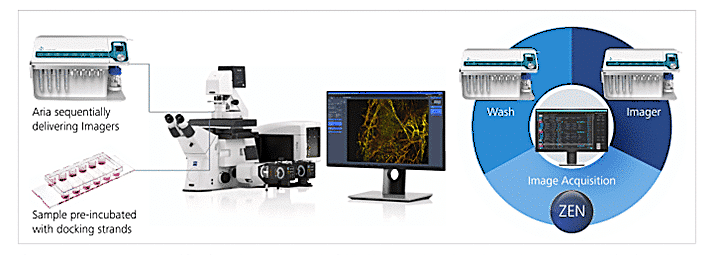
Workflow Technical Parameters
- Imager concentrations (example): DNA origami: ~1.5 nM imagers (Cy3B). U2OS cells: R3 = 300 pM, R4 = 250 pM (Cy3B).
- Buffer additives: Trolox, PCA, PCD oxygen scavenging system to protect docking sites and improve blinking of fluorophores.
- Fiducials: 90 nm gold nanoparticles used for drift correction and channel alignment.
- Typical workflow: Cycle = wash (PBS) → deliver imager → image acquisition → wash → next imager. Loop blocks in Aria run 2–4 iterations depending on sample.
- Post-processing: Localization reconstruction with Picasso (Jungmann lab). Drift correction via fiducials and cycle registration required to build multi-channel image.
- Performance achieved: Localization precision ~4 nm (origami) and ~5 nm (cells); final mean resolution ~9 nm (origami) and ~12 nm (U2OS).
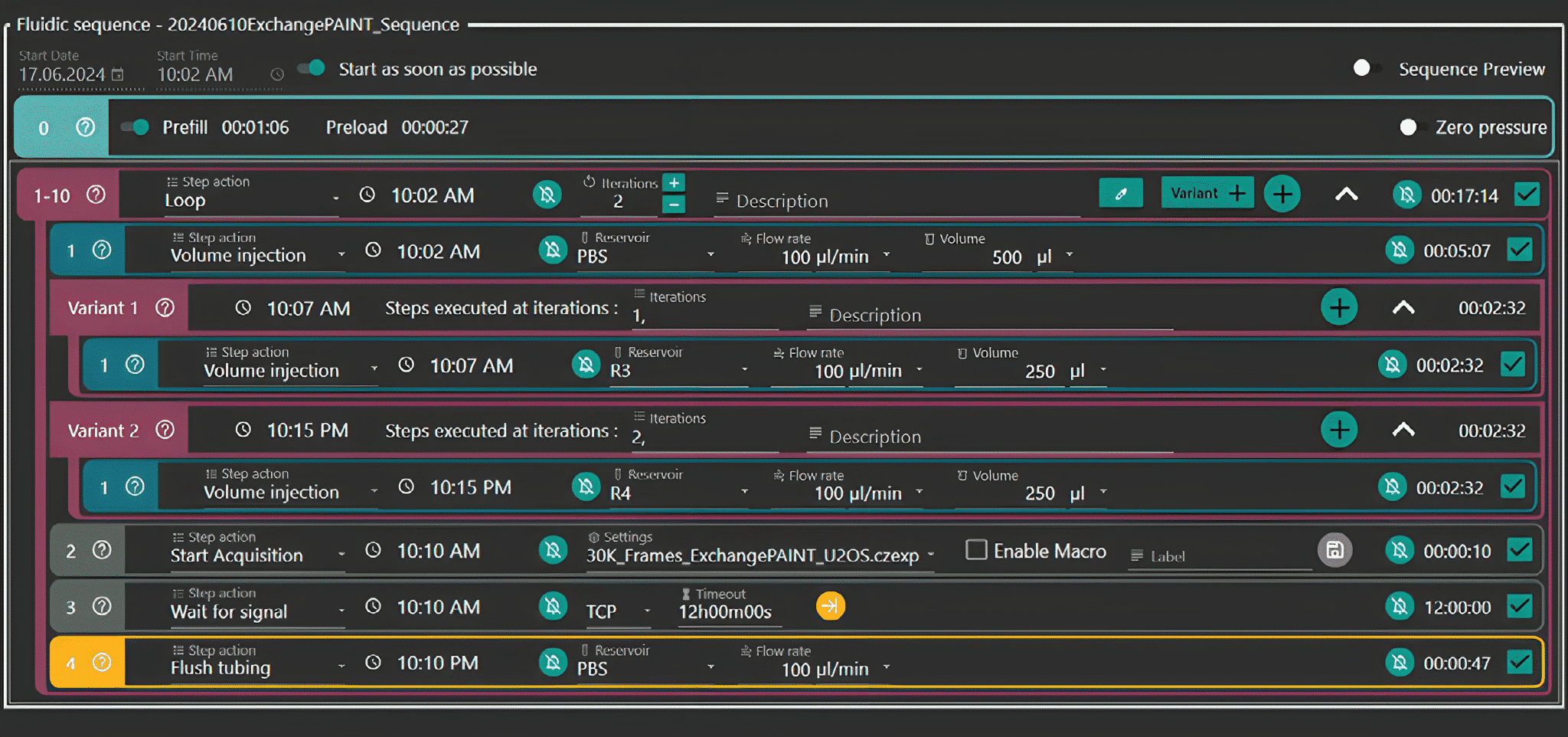
Figure 3: Aria Workflow for Echange-Paint on cultured cells. With integration of acquisition protocol (“30K_Frames_Origami.czexp”)
Overview of Other Cyclical Multiplexing Methods vs Exchange-PAINT
The basic steps between staining and washing steps are compared to other iterative methods:
| Method | Type of Label | Imaging Modality | Signal Removal Method | Details |
|---|---|---|---|---|
| IBEX (Iterative Bleaching Extends multiplexing) | Fluorophore-conjugated antibodies | Confocal / Widefield | Chemical bleaching (e.g., LiBH₄) | Relatively fast cycles; bleaching may cause tissue damage after many cycles. |
| t-CyCIF (Cyclic Immunofluorescence) | Fluorophore-conjugated antibodies | Confocal / Widefield | Chemical bleaching (e.g., LiBH₄) | Relatively fast cycles; bleaching may cause tissue damage after many cycles. |
| 4i (Iterative Indirect Immunofluorescence Imaging) | Primary + fluorophore-conjugated secondary antibodies | Confocal / Widefield | Antibody elution (e.g., low-pH or SDS) | Uses mild antibody elution to remove all antibodies; excellent tissue preservation; multiple cycles with minimal antigen loss. |
| MERFISH / seqFISH | Fluorescently labeled RNA probes (barcoded) | Confocal / Widefield | Formamide stripping / DNase digestion | In situ RNA imaging via sequential rounds of hybridization and signal removal |
| CODEX (Co-Detection by Indexing) | DNA-barcoded antibodies + fluorescent reporters | Epifluorescence / Spinning Disk | DNA strand removal / washout | DNA barcodes are hybridized with labeled oligos, then washed out between cycles. Fast cycles, wash cycles |
| DNA-PAINT Microscopy / Exchange-PAINT | DNA-tagged probes + imager strands | TIRF / Super-resolution | Imager strand washout | DNA strands transiently bind; changing the strand allows cycling without bleaching. Requires precise fluid exchange. |
Download the full application note
Learn more about post-processing, data correction methods, and detailed workflows in the full ZEISS application note (PDF).
The benefits of Aria in Exchange-PAINT:
- Microfluidic systems like Aria precisely deliver imager reagents to the sample on-stage
- Automated fluid delivery system saves time by reducing hands-on operation time
- The field of view remains aligned between consecutive imaging rounds.
- Workflow setup with intermittent imaging steps is simple with Aria + Elyra 7, as Aria can directly control ZEN image acquisitions.
- Final localization precision in the reported experiments was ~5 nm, fully within expected performance for DNA-PAINT.
Related product
Webinar that might interest you
Related Resources
-
Microfluidics Case Studies Multi-parametric Functional Assays of Cardiac Organ-on-a-Chip using Live-cell Microscopy and Fluigent, Aria Read more
-
Expert Reviews: Basics of Microfluidics Optimizing Microfluidic Perfusion: Best Practices and Innovations Read more
-
Microfluidic Application Notes Automating calcium imaging in neural cells with Fluigent’s Aria Read more
-
Interviews & Testimonials Users Testimonials – They trust Fluigent’s Instruments Read more
-
Microfluidics Case Studies University of Rochester: A tissue chip platform for real-time sensing of secreted inflammatory markers using ARIA Read more
-
Microfluidic Application Notes Automated Immunofluorescence using Aria Read more
-
Microfluidics Case Studies Automated immunolabeling to perform highly multiplexed tissue imaging with ARIA Read more
-
Microfluidics White Papers A review of Organ on Chip Technology – A White Paper Read more