Liposome Nanoparticle Synthesis
In this application note, the Raydrop (developed and manufactured by Secoya) is used to perform Liposome Nanoparticle synthesis.
Secoya developed and manufactured the RayDrop used to perform this application note.

Introduction
Despite considerable progress in recent years, various disease diagnostics and treatments continue to present constraints such as low sensitivity or specificity, drug toxicity, and severe side effects [1]. Cancer represents one of the best examples of a disease where localized delivery of therapeutics is of great importance, as the potent yet toxic mechanisms of action of such compounds can lead to either an effective response or side effects. Today, most drug formulations are not capable of targeting specific sites of interest. Nanoparticle-based drug delivery platforms have emerged as suitable vehicles for overcoming these limitations [2]. Nanoparticles, such as liposomes, have proven advantageous at preserving therapeutic material and allowing for extended half-lives of drugs within the body [3].
Liposome nanoparticles are specialized delivery vehicles that serve multiple roles in enhancing the capabilities of active pharmaceutical ingredients (APIs). They can shield a drug from detection by the body’s immune system, and they serve to help solubilize highly lipophilic drug molecules or modulate the pharmacokinetics and biodistribution of the API.
What are liposome nanoparticles?
Liposome nanoparticles were discovered in the 1960s. These hollow nanoparticles are phospholipid vesicles consisting of at least one lipid bilayer (figure 1). This bilayer is usually composed of amphiphilic phospholipids that have a hydrophilic phosphate head and a hydrophobic tail consisting of two fatty acid chains. This structural feature has facilitated liposome applications, including their use as artificial cell membranes, carriers for drug delivery systems, encapsulating agents for food ingredients, and analytical tools [4–8].
Recent state-of-the-art applications
During the COVID-19 pandemic, the first vaccines to reach clinical trials were based on viral vector and nucleic acid technologies. One of the most promising vaccine candidates was based on nucleoside-modified mRNA and encapsulated within lipid nanoparticles (LNP) [9]. This confirms the need for lipidic nanoparticles for present and future drug delivery applications.
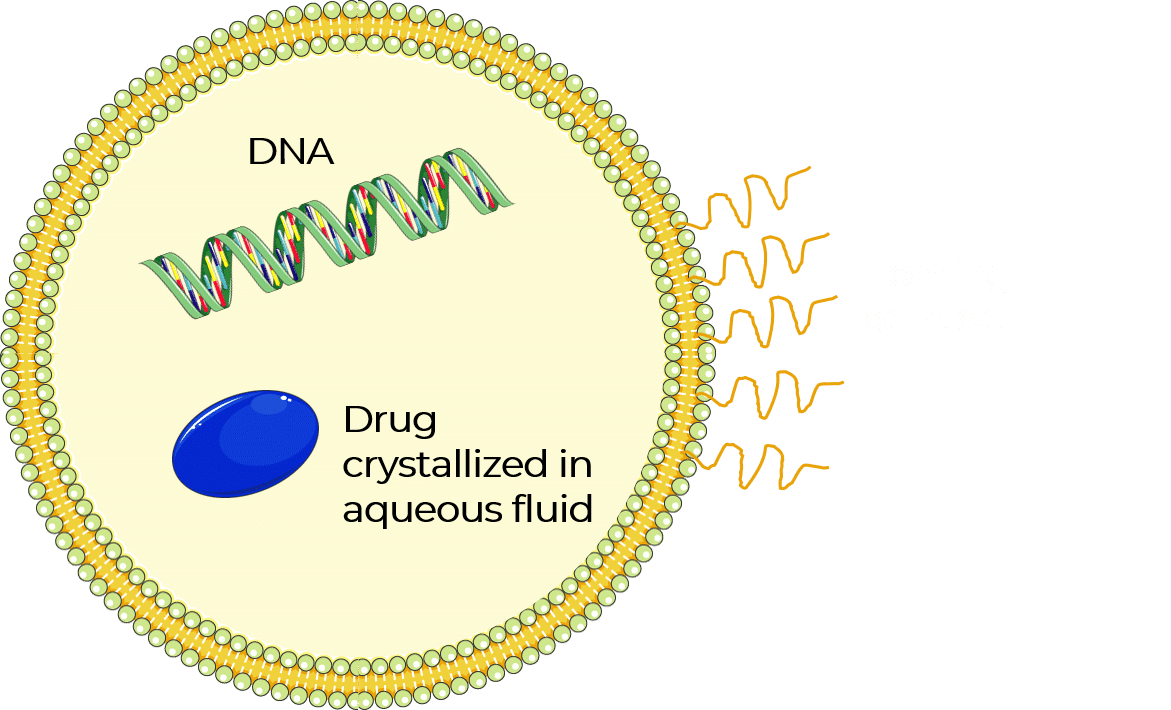
In recent years, liposome nanoparticles have attracted significant attention as a trusted class of drug delivery vehicles. Their self-closed structures can encapsulate multiple drugs at once, protecting the enclosed cargo from hydrolysis and breakdown. Additionally, targeting proteins and surface functional ligands on the outer shell of the lipid bilayer can add novel functionality—enabling targeted entry of liposomes into cells, either via antibodies or receptor-targeted ligands. These ligands attach to cell receptors that are over-expressed in certain diseased cells, allowing entry of the drug through the cell membrane.
What is the difference between Liposome and Lipid Nanoparticles?
Liposome and lipid nanoparticles are lipid-based structures used for drug delivery. Liposomes are spherical vesicles with lipid bilayers surrounding an aqueous core, while lipid nanoparticles are solid particles composed of solid lipids or a mixture of solid and liquid lipids. Liposomes have a hydrophilic outer layer and can encapsulate both hydrophilic and hydrophobic molecules, offering versatility but potentially reduced stability.
In contrast, lipid nanoparticles provide improved stability and are relatively easier to manufacture at scale. They are especially suitable for encapsulating poorly water-soluble drugs or nucleic acids. Liposomes have been widely used, while lipid nanoparticles are gaining in popularity, particularly in mRNA-based vaccines like those for COVID-19. Both lipid-based systems contribute to advanced drug delivery strategies, with liposomes offering flexibility and lipid nanoparticles providing enhanced stability and ease of production.
Comparison with Another Production Method
| Batch method | Fluigent microfluidic method | |
| Particle size distribution | Low | High |
| Reproducibility | Low | High |
| Live particle size control | No | Precise |
| Range of particle size | Limited size range | Wide size range |
| Continuous (/in line) production | No | Yes |
How to produce liposome nanoparticles
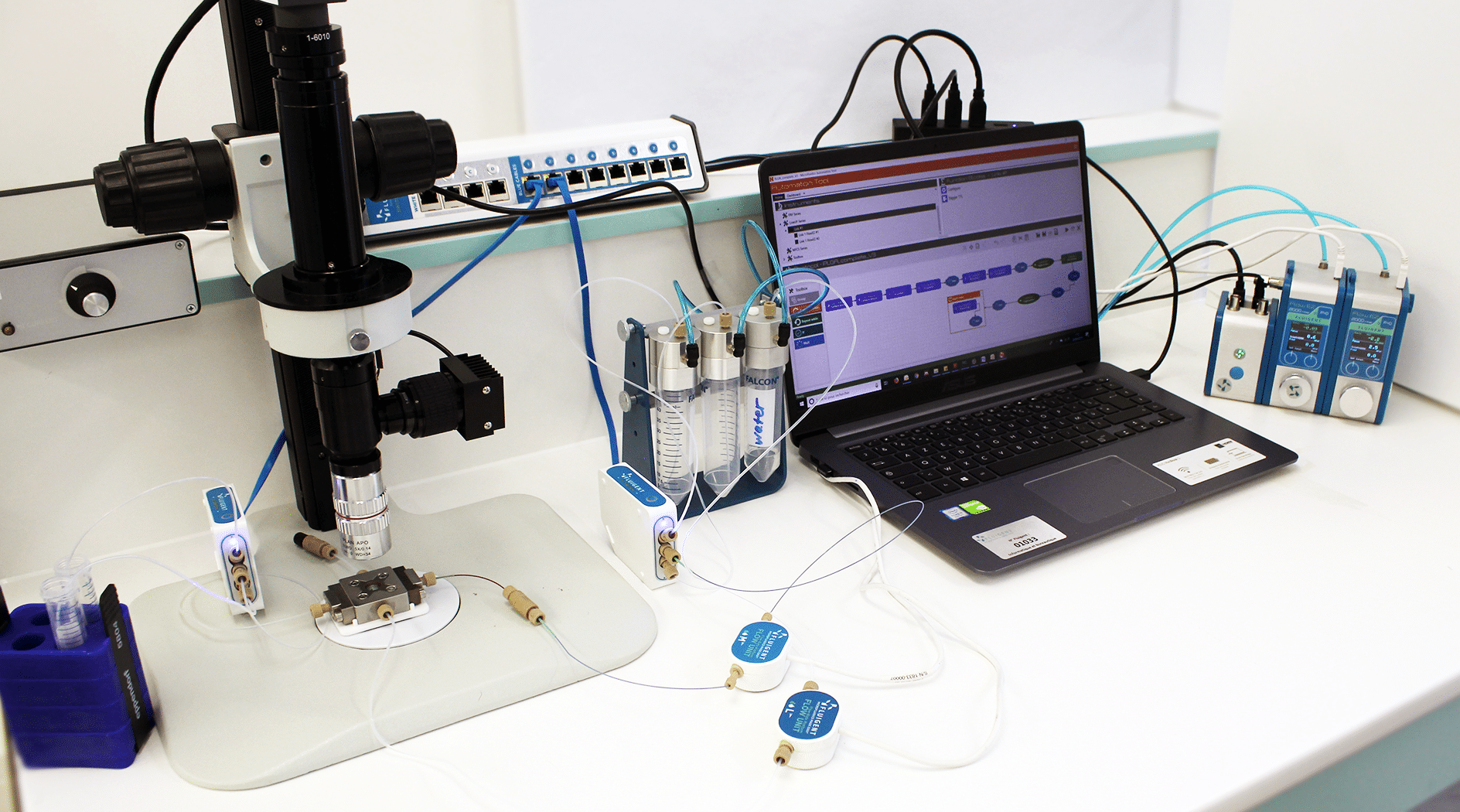
Equipment
Liposome nanoparticle generation
A stream of lipid in alcohol solution is surrounded by an aqueous phase within a glass capillary. The alcohol solution containing lipids diffuses into the aqueous solution (and reciprocally the water diffuses into the alcohol), until the alcohol concentration decreases to the solubility limit of the lipids. Consequently, this diffusion triggers the formation of liposomes by a mechanism described as “self-assembly”, where lipids assemble into a more energetically favorable structure.
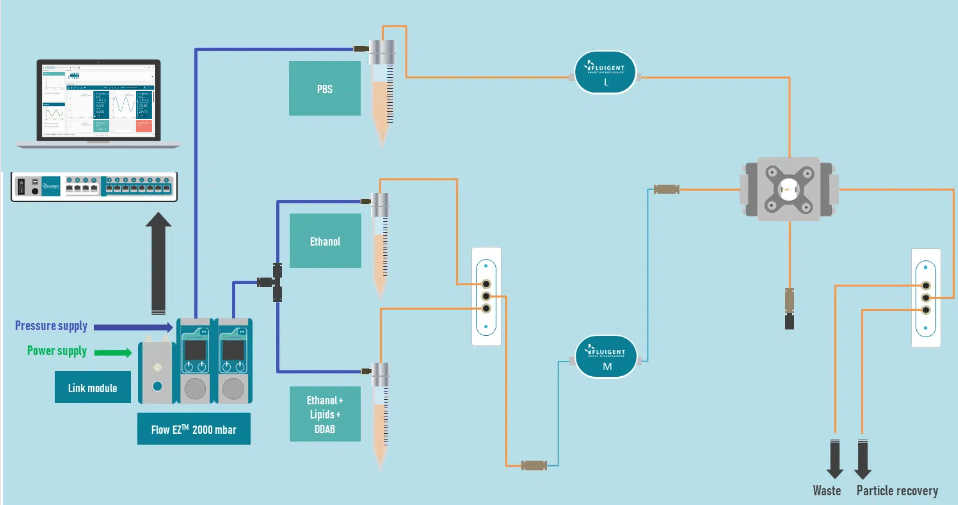
The liposome nanoparticle production system is illustrated in figure 2.
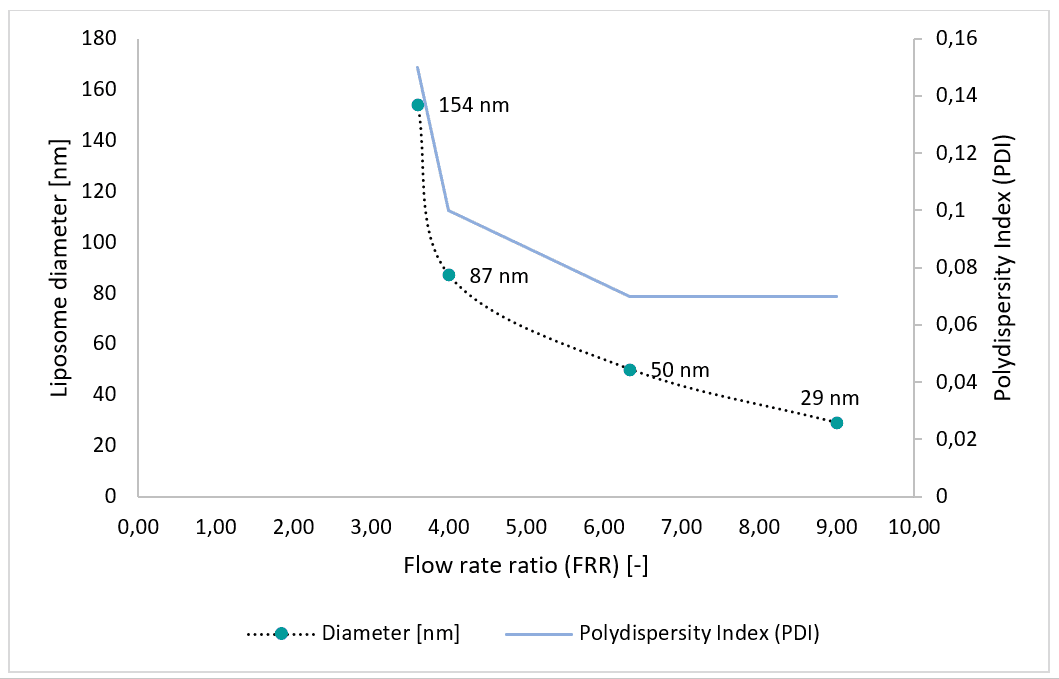
Partial results
Conclusion
Liposome nanoparticles have proven advantageous for solubilizing therapeutic substances. Macroscale batch methods widely employed for liposome production lack control over liposome morphology, size, and distribution. Microfluidic systems allow for the production of highly monodisperse liposome nanoparticles. We have demonstrated the production of liposomes using a microfluidic system consisting of pressure-based flow controllers and the Raydrop™ microfluidic device with standard configuration. Liposomes ranging from 30 to 150 nm were generated. Sizes can be adjusted by controlling the device flow input parameters, particularly the flow rate ratio (FRR). The polydispersity index (PDI) ranges from 0.07 to 0.15. This system enables synthesis of liposomes for drug delivery applications, as encapsulating agents for food ingredients, or for other applications requiring nano-sized and spherical liposomes.
A complete, cost-effective and commercially-available platform for on-demand production of monodisperse liposome nanoparticles is now available. This allows for control of liposome size and frequency by adjusting flow parameters.
Related Resources
- Microfluidics Article Reviews
Microfluidic technology for engineered nanoparticles in nanomedicine
Read more - Fluigent products manual
Liposome nanoparticles production station Protocol
Download - Fluigent Products Datasheets
Liposome Production Pack Datasheet
Download - Fluigent products manual
Liposome Production Pack User Guide
Download - Fluigent Products Datasheets
PLGA Nanoparticle Production Station Datasheet
Download - Expert Reviews: Basics of Microfluidics
Microfluidics for vaccine development
Read more - Microfluidics White Papers
Droplet-based Microfluidics – A Complete Guide
Read more
References
- Bozzuto, G. & Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomedicine 10, 975–999 (2015).
- Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
- Torchilin, V. P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 4, 145–160 (2005).
- Bally, M. et al. Liposome and lipid bilayer arrays towards biosensing applications. Small 6, 2481–2497 (2010).
- Fathi, M., Mozafari, M. R. & Mohebbi, M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Technol. 23, 13–27 (2012).
- Grimaldi, N. et al. Lipid-based nanovesicles for nanomedicine. Chem. Soc. Rev. 45, 6520–6545 (2016).
- Andrew Pohorille & David Deamer. Artificial cells: prospects for biotechnology. Trends Biotechnol. Biotechnol. 31- (2002).
- Rongen, H. A. H., Bult, A. & Van Bennekom, W. P. Liposomes and immunoassays. J. Immunol. Methods 204, 105–133 (1997).
- Vogel, A. B. et al. A prefusion SARS-CoV-2 spike RNA vaccine is highly immunogenic and prevents lung infection in non-human primates. bioRxiv 2020.09.08.280818 (2020).
- Pattni, B. S., Chupin, V. V. & Torchilin, V. P. New Developments in Liposomal Drug Delivery. Chem. Rev. 115, 10938–10966 (2015).
- Mui, B., Chow, L. & Hope, M. J. Extrusion Technique to Generate Liposomes of Defined Size. Methods Enzymol. 367, 3–14 (2003).
- Carugo, D., Bottaro, E., Owen, J., Stride, E. & Nastruzzi, C. Liposome production by microfluidics: Potential and limiting factors. Sci. Rep. 6, 1–15 (2016).
- Hood, R. R., Devoe, D. L., Atencia, J., Vreeland, W. N. & Omiatek, D. M. A facile route to the synthesis of monodisperse nanoscale liposomes using 3D microfluidic hydrodynamic focusing in a concentric capillary array. Lab Chip 14, 2403–2409 (2014).
- Jahn, A., Vreeland, W. N., Devoe, D. L., Locascio, L. E. & Gaitan, M. Microfluidic directed formation of liposomes of controlled size. Langmuir 23, 6289–6293 (2007).
- Jahn, A. et al. Microfluidic mixing and the formation of nanoscale lipid vesicles. ACS Nano 4, 2077–2087 (2010).