Liver–Kidney Organ-On-Chip Model using the Omi™ Dual Platform
Tacrolimus is a commonly used immunosuppressant for liver transplant patients, but its use is limited by a narrow therapeutic window, variable pharmacokinetics, and risk of nephrotoxicity. To better understand how the liver and kidney jointly process this drug, researchers developed a interlinked organ-on-chip (OOC) model using the Omi™ Dual Platform. This system combines HepaRG spheroids and RPTEC/TERT1 cells in a dynamic microfluidic circuit, allowing real-time study of hepatic metabolism and renal transporter activity under continuous recirculation.
Over 48 hours, the platform revealed rapid hepatic metabolism, limited renal clearance aligned with biliary excretion, and the presence of tacrolimus metabolites across compartments. Complementary analyses, such as mRNA quantification, immunofluorescence, LC-MS/MS, and metabolomics, confirmed model function and distinct organ-specific metabolic responses. Together, these findings position the Omi™ Dual Platform as a relevant tool for studying pharmacology and drug–drug interactions.
This application note is written in collaboration with University of Limoges Pharmacology and Transplantation UMR 1248.
Download the full application note to explore the data, detailed results, and implications for advancing pharmacology and drug–drug interaction research.

Understanding Hepatic and Renal Interactions with OOC Technologies
Renal dysfunction following liver transplantation is frequently attributed to tacrolimus therapy, highlighting the necessity for advanced models that capture inter-organ pharmacology. The liver and kidney together govern the disposition of tacrolimus via hepatic metabolism (e.g. CYP3A4/5), biliary excretion, and renal transporter-mediated uptake/efflux (Figure 1). However, conventional in vitro and in vivo systems often lack the capacity to faithfully recapitulate organ crosstalk and dynamic drug flow.
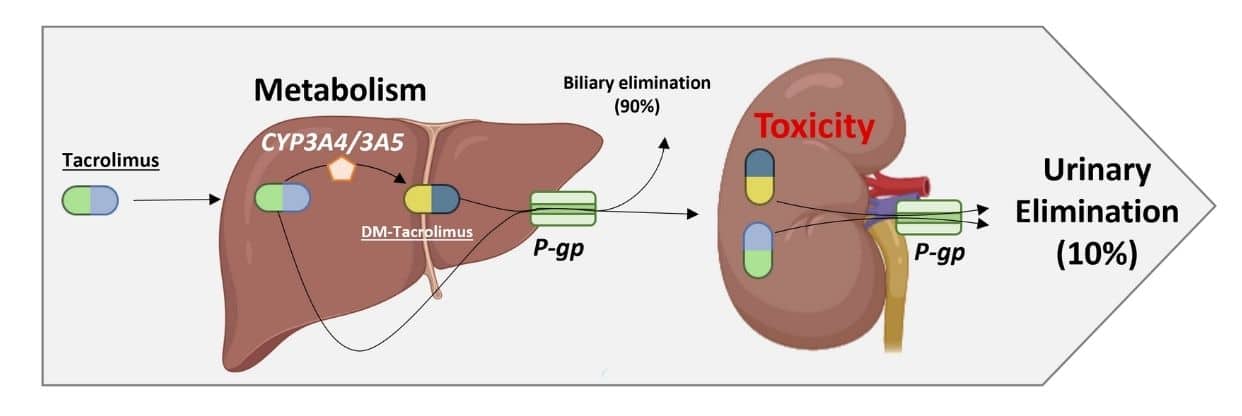
Figure 1. Tacrolimus pharmacology: liver and kidney involvement. Illustration showing tacrolimus metabolism by hepatic CYP3A4/5 enzymes, biliary elimination (~90%), minor urinary excretion (~10%), and P-gp–mediated efflux in both liver and kidney compartments.
Why a Liver–Kidney OOC Model Matters in Pharmacology Research
Organ-on-chip (OOC) technologies provide a solution that enables the control of physiological microenvironments under fluidic flow. Single-organ chips are used to model and to access specific parameters like the toxicity, the effect of shear stress, vascular interactions, cancer treatments etc. While linked, multi-organ models allow investigation of cross-organ response. More recently, reviews emphasized the growing application of multi-organ OOC for ADME, drug-drug interactions and toxicity testing with higher translational relevance than in vivo or static models[1]
Kidney-specific models are focused on the reproduction of key physiological conditions of renal proximal tubule active transporter function and metabolic gradients. When combined with liver-on-chip systems, such integrated multi-organ models allow for the investigation of how hepatic metabolism influences renal exposure and potential toxicity.
Building the Liver–Kidney Dual OOC Model: Materials and Methods
- Cell culture: RPTEC/TERT1 proximal tubule cells and HepaRG spheroids cultured in Be-Doubleflow and µSlide devices to model renal and hepatic compartments.
- Omi Dual Mode: Automated dual recirculation at 10 µL/min for the renal compartment and 20 µL/min for the hepatic compartment for 48 hours to mimic physiological perfusion.
- mRNA quantification: qPCR analysis to evaluate transporter and receptor expression changes.
- Immunofluorescence: Protein localization and function validation in HepaRG spheroids.
- LC-MS/MS compound quantification: Measurement of tacrolimus and its metabolites in culture media.
- Metabolomics: Comprehensive profiling of metabolic alterations induced by treatments.
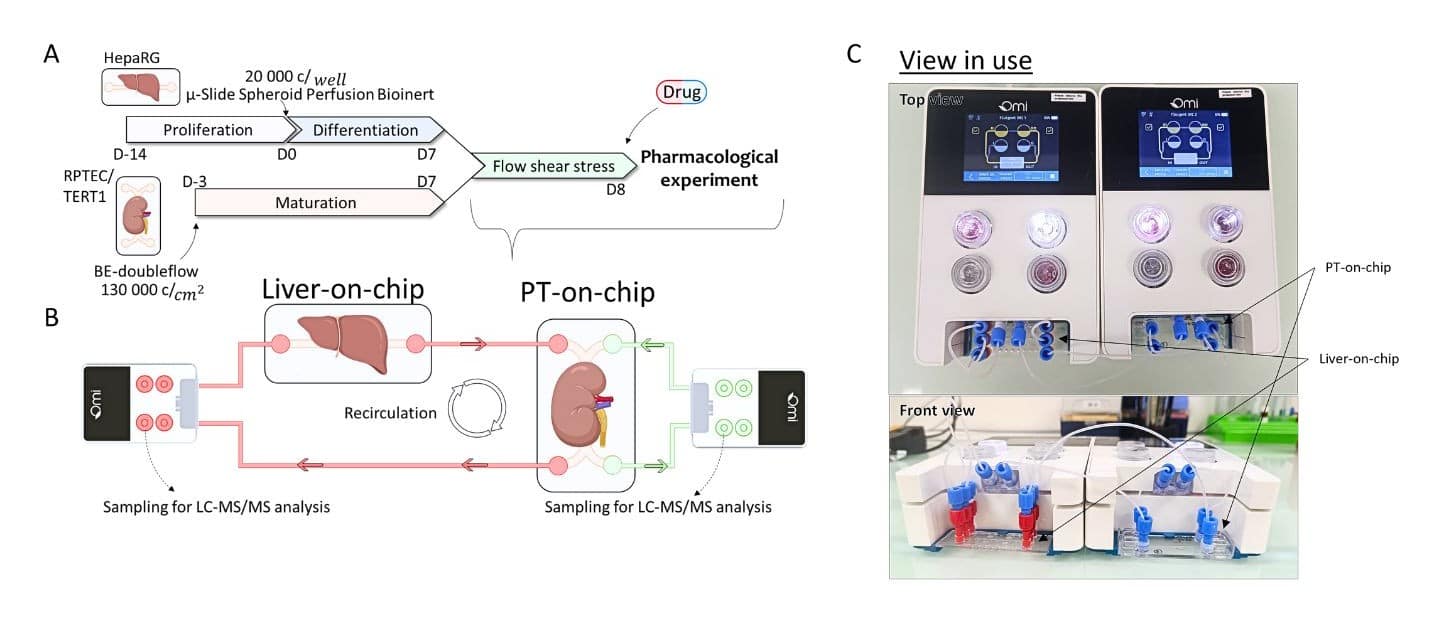
Figure 2. Overview of Liver-Kidney Dual OOC setup
Key Findings from the Liver–Kidney Organ-On-Chip Model
Tacrolimus displayed a rapid decrease in concentration within the hepatic compartment, stabilizing after approximately eight hours, consistent with active uptake and metabolism by HepaRG cells and limited renal excretion. The drug appeared later in the urinary compartment, peaking after ten hours at roughly one-tenth of the hepatic concentration, consistent with its in vivo biliary-dominant clearance. Detection of the metabolite DM-tacrolimus in both compartments confirmed efficient hepatic metabolism and partial transfer of metabolites to the renal side, demonstrating that the dual-organ system effectively reproduces key aspects of tacrolimus disposition and elimination.
At the transcript level, tacrolimus alone did not alter PXR expression, while combination with metformin produced a modest increase in both renal and hepatic cells. Transporter genes ABCB1/P-gp and ABCC4/MRP4 remained stable, whereas ABCC2/MRP2 showed mild downregulation under tacrolimus exposure.
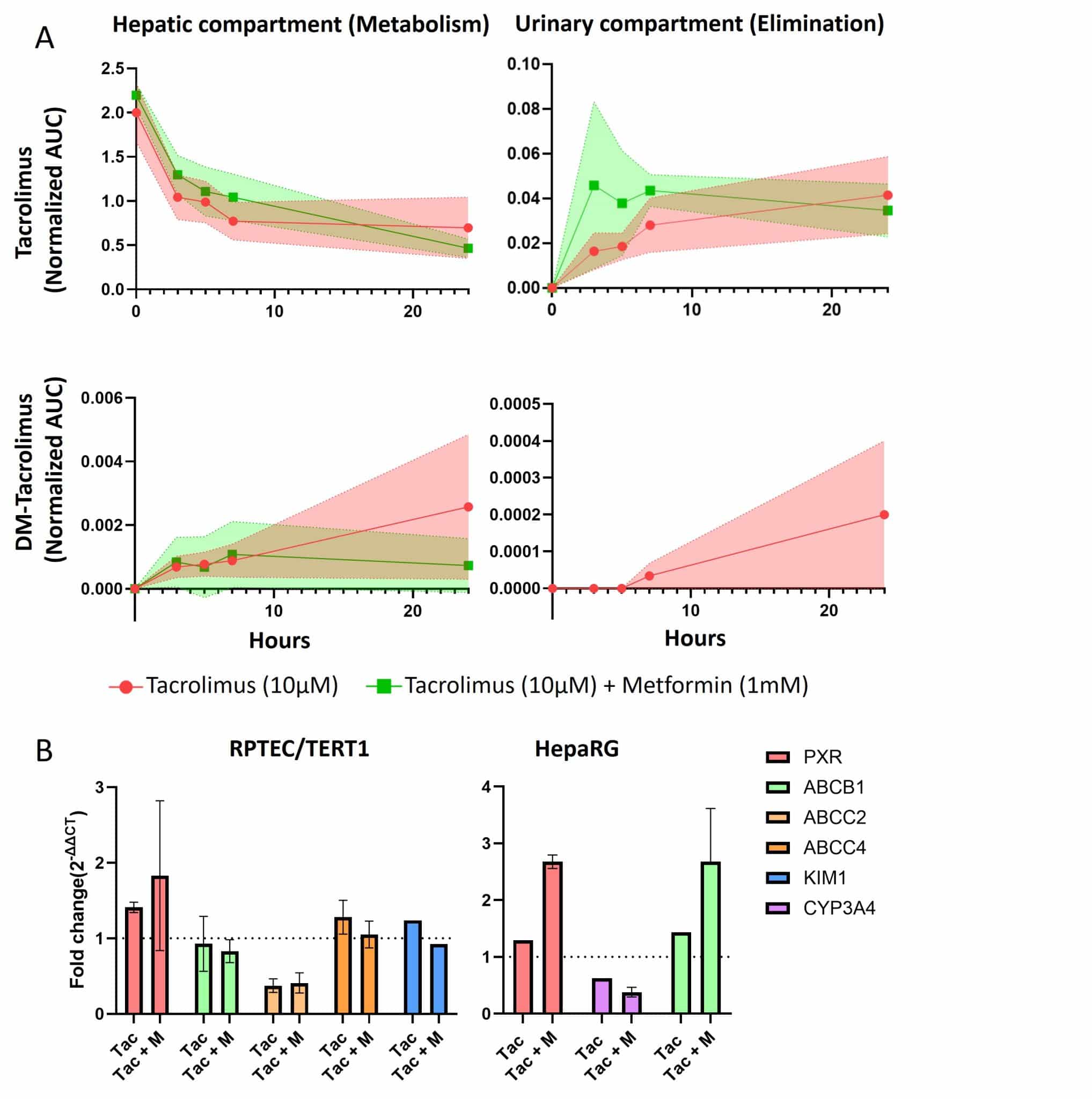
Figure 3. Tacrolimus effect onto the dual-organ models. (A) Area under the curve (AUC) for tacrolimus on the top panels and DM-tacrolimus on the bottom panels in the hepatic compartment on the left and the urinary compartment on the right (n= 3 for each condition) during tacrolimus (10µM) treatment (red) and tacrolimus (10µM) + metformin (1mM) co-treatment (green) the color area represent the error bar range.
Download the full application note to explore the complete dataset, experimental details, and insights into how dual organ-on-chip models can transform drug development and safety assessment.
Fluigent Author: Anel Rakhmatullina
Related Solutions
Related content
-
Microfluidics Case Studies Gut-on-Chip Modeling: From Chip Development to Perfusion Read more
-
Expert Reviews: Basics of Microfluidics Optimizing Microfluidic Perfusion: Best Practices and Innovations Read more
-
Expert Reviews: Basics of Microfluidics Microfluidics in Drug Delivery: A New Era of Precision Medicine Read more
-
Microfluidic Application Notes Gut-on-Chip Model Development Using OOAC Platform, Omi Read more
-
Microfluidics Case Studies Creating kidney organoids‑vasculature interaction model using Fluigent’s Flow-EZ Read more
-
Microfluidics Case Studies CNRS/UTC: study of a liver-on-a-chip model Read more
-
Microfluidics White Papers A review of Organ on Chip Technology – A White Paper Read more