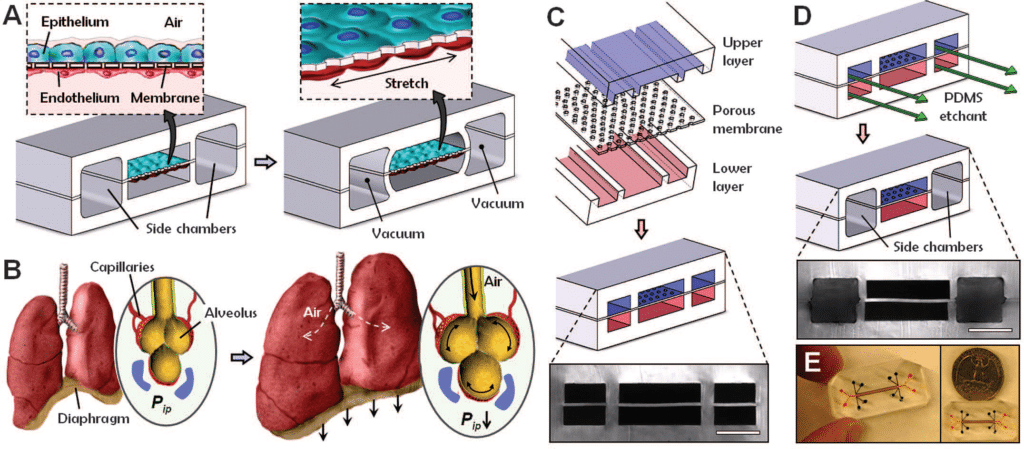
General Overview of Microfluidics
Microfluidics is a multidisciplinary field of science and technology that deals with the behavior, control, and manipulation of very small volumes of fluids, typically in the microliter (10^-6 liters) to picoliter (10^-12 liters) range.
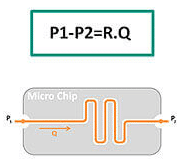
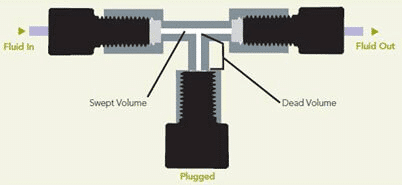
To perform effective experiments in microfluidics, one needs to be aware of the different volumetric control technologies available to use the most suitable way to control microfluidic flows. This section will give you all the information regarding Microfluidic Volume Definition and Resistance, but also on the Strengths and Weaknesses of the different flow control technologies existing on the market.