Microfluidic Temperature Control module
A variety of microfluidic applications are performed at controlled temperature, including organs on chip applications and live cell imaging for localization microscopy, as well as droplet microfluidics for digital PCR, cell encapsulation, and droplet polymerization. These applications are highly dependent on the applied temperature, and therefore require fine temperature control. However, this control is usually not integrated, thereby limiting the potential applications. Heating and temperature control modules can be developed according to the application.
Fluigent has developed microfluidic temperature control modules that can be coupled to microfluidic systems to maintain microfluidic components at any desired temperature. Visit our technology webpage to learn more about the working principles of our temperature control module and how it can be integrated into your microfluidic system.
This technology can be integrated into any custom project.
- High performance: stability +/- 0,2°C
- User friendly: easily switch between heating and cooling modes
- Versatile: can be integrated into Fluigent’s protocols and communicate with other modules in a larger microfluidic setup
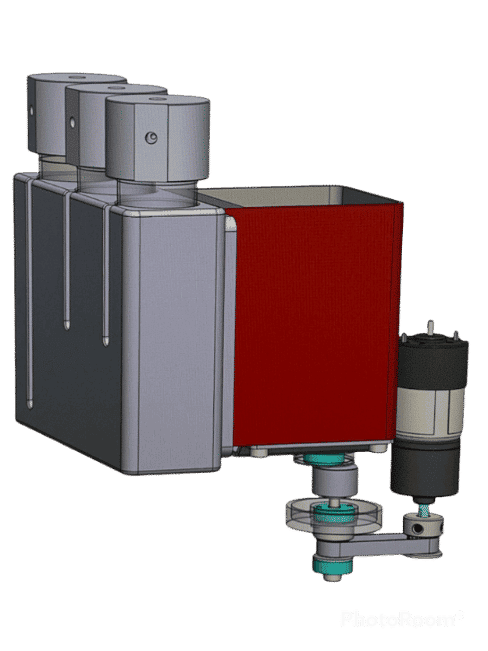
How does our microfluidic temperature regulation system work?
Fluigent’s microfluidic temperature control module
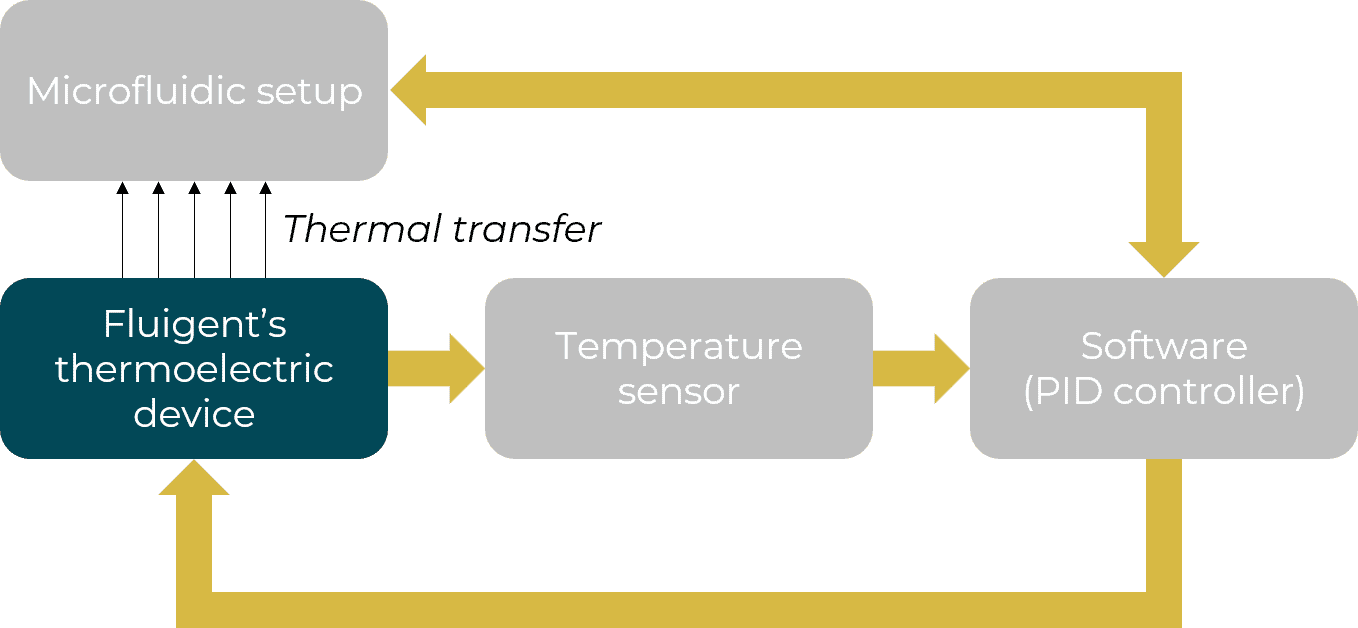
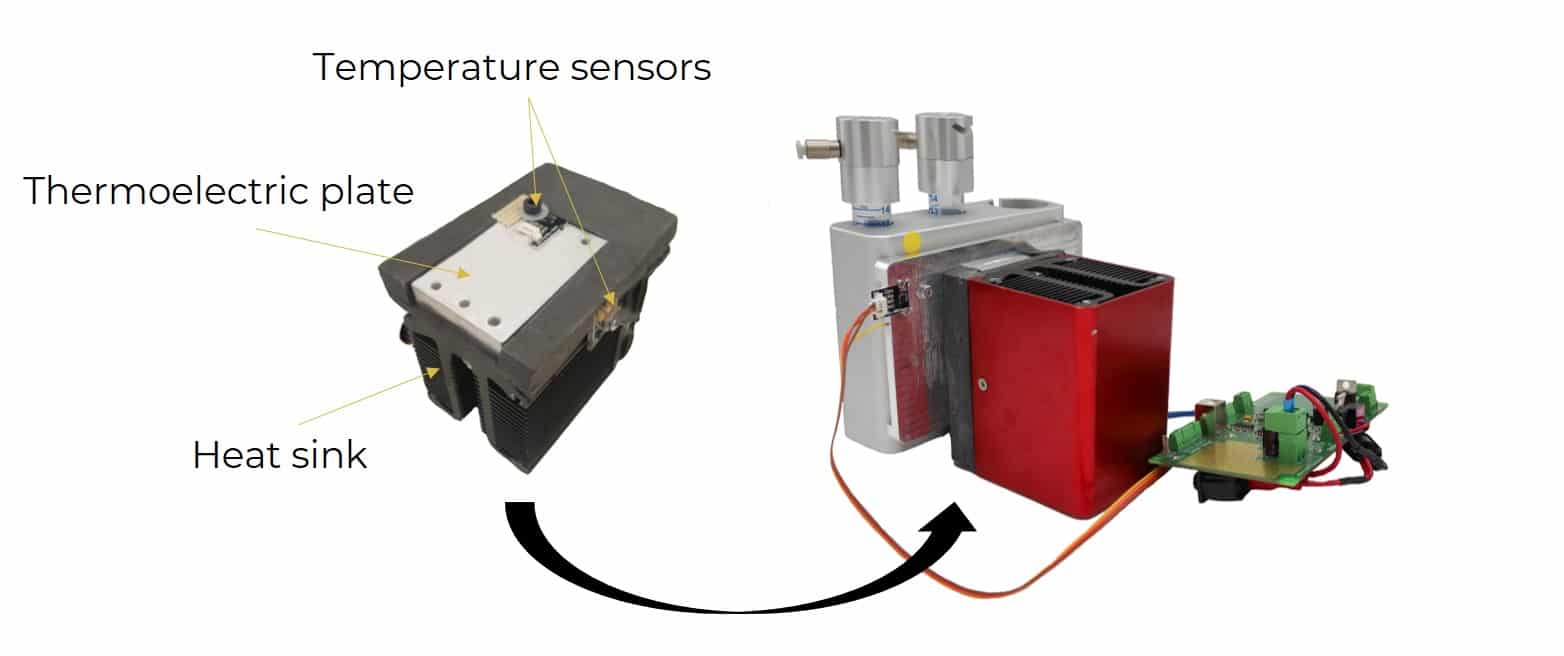
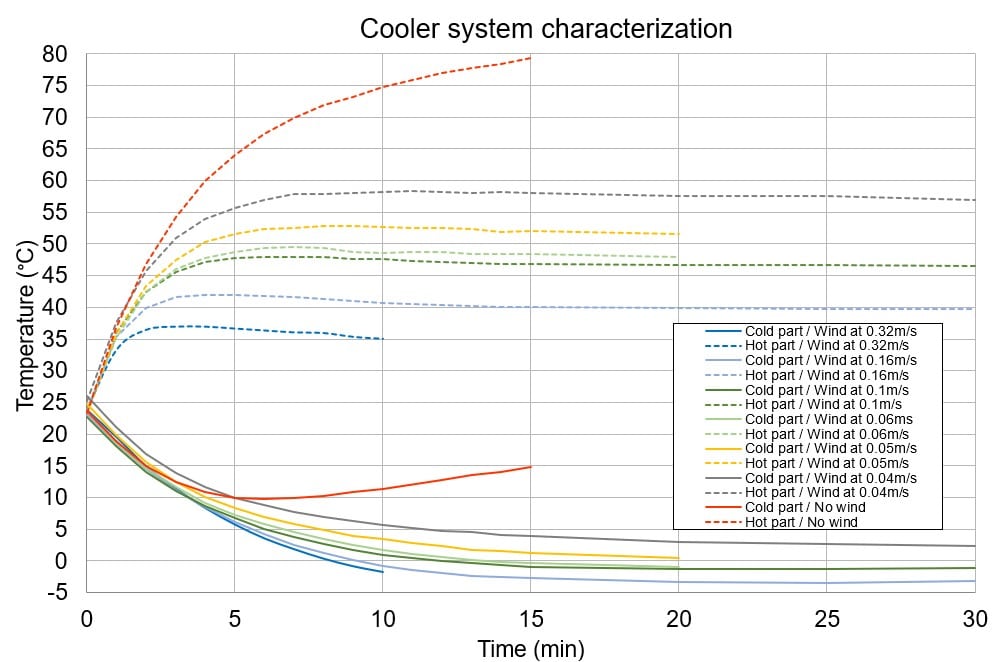
The temperature controller is based on direct conversion of the electric current into thermal energy. It allows the temperature to be close to 0°C on one side of a thermoelectric device. On the other side, a straight-fin heat sink helps with heat dissipation. The goal of the heat sink is to maximize the surface within a fixed volume to optimize the heat exchange between the device and ambient air. It can be coupled with a fan to be more efficient. Sensors are placed on each side to monitor the temperature change.
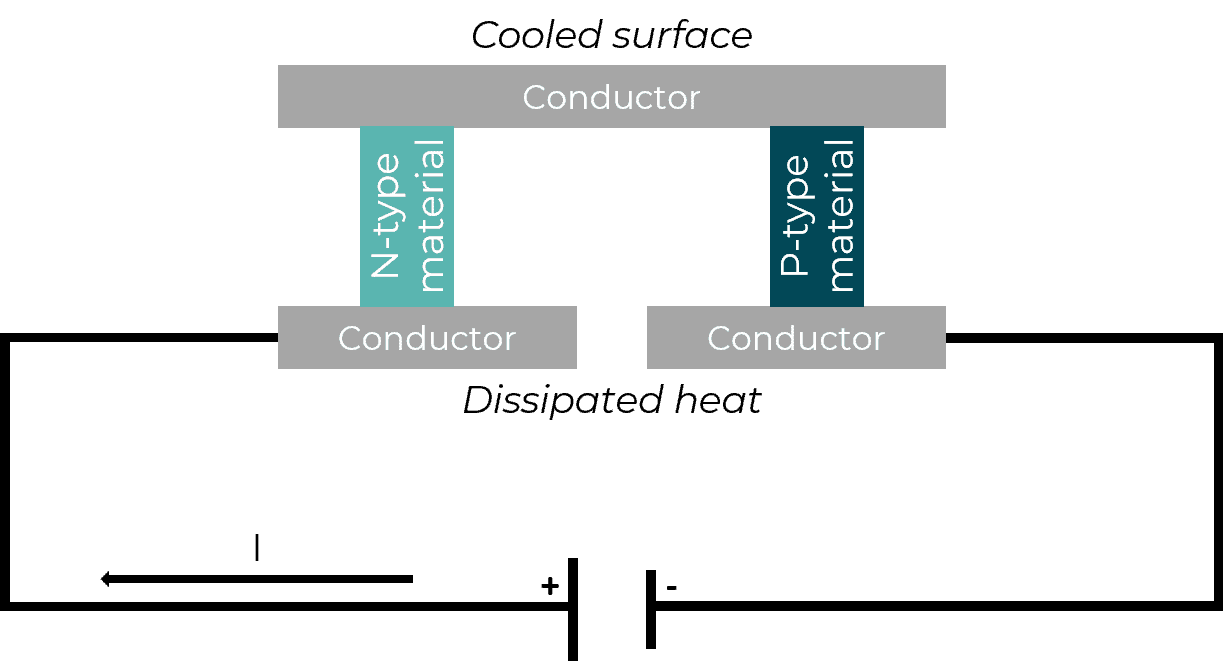
Microfluidic temperature control benefits of Peltier elements
The Peltier effect is used as a solid-state method of inducing small temperature increases in devices known as thermoelectric plates or Peltier elements. When DC current flows through, heat is transferred from one side of the device to the other. The scheme below shows how cooling works in these systems. In contact with the cooled surface, samples are maintained at low temperatures, while on the other side of the device, resistive heating generated by current flow leads to emission of heat. [1]
Using semiconductors in a thermoelectric plate saves power, consuming only 10% of the power needed by a traditional air conditioner to achieve the same temperature. Moreover, thanks to its compact and lightweight form factor, it is a cooling technique well suited to microfluidic applications. Lastly, it is durable, has no mechanical moving parts, and does not require contact with fluids. [2]
Fluigent’s microfluidic temperature management expertise equals performance
Stability and accuracy
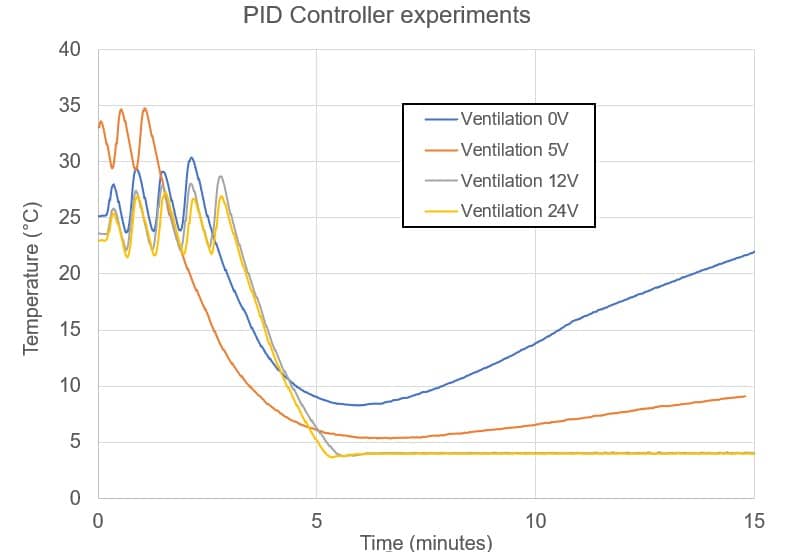
The use of ventilation enables the temperature control module to reach the set temperature, and the PID controller prevents overrunning and oscillations. With the right adjustment of air speed and control loop parameters, the temperature control module stays within a margin of error of 0.2°C of the set value once the transition phase is complete.
Response Time
The set temperature is reached in less than 7 minutes, depending on air circulation. Applications usually do not require a specific response time, but it could be improved with greater intensity of the electric current, stronger ventilation and/or calibration of the PID controller in the microfluidic temperature control module.
Applications requiring microfluidic temperature regulation
- Cell encapsulation
Cell encapsulation is used to improve the recovery of viable cells, a highly temperature-dependent process. As refrigeration is the preferred method for short-term storage of cells, it is crucial to maintain the samples before encapsulation below 5°C during the entire duration of the experiment in order to preserve them. [3][4]
- Live cell imaging
At ambient temperatures, fluorescent microscopy may be affected by photo-bleaching for certain specific experiments. However, this drawback is reduced at low temperatures. The number of photons emitted by fluorescent molecules can also increase, and the signal to noise ratio of fluorescence imaging can improve. [5][6]
Moreover, the ability to quickly and reversibly heat cells is useful in the study of the cell cytoskeleton, which plays an essential role in several cellular processes, because its dynamic microtubule responds to temperature changes in the range of 2-50°C induced by Peltier elements. [7][8][9]
- Droplet Production
Temperature impacts various parameters during microfluidic-based droplet generation. For instance, the size and frequency of formation of droplets increase with temperature. The regularity of their shape and the flow regime also depends on the microfluidic temperature control of the dispersed and continuous phases.
- Temperature gradient focusing
With two Peltier elements separated by a small gap, one can create a temperature gradient across a microchannel or a capillary. Combined with an applied electric current and an appropriate buffer, it can concentrate analytes by balancing the electrophoretic velocity of the analytes against the bulk flow. It can also separate them, thanks to the electrophoretic velocity gradient generated by the temperature gradient. [12][13]
Related products
Expertises & resources
References
[1] S. B. Riffat, X. Ma, “Improving the coefficient of performance of thermoelectric colling systems: a review”, International Journal of Energy Research, 2004
[2] S. Kumar, A. Gupta, G. Yadav, H. P. Singh, “Peltier Module for Refrigeration and Heating using Embedded system”, International Conference on Recent Developments in Control, Automation and Power Engineering, 2015
[3] S. Swioklo, A. Constantinescu, C. J. Connon, “Alginhate-Encapsulation for the Improved Hypothermic Preservation of Human Adipose-Derived Stem Cells”, Stem Cells Translational Medecine, 2016
[4] A. Z. Khan, T. P. Utheim, C. J. Jackson, K. A. Tonseth, J. R. Eidet, “Concise Review: Considering Optimal Temperature for Short-Term Storage of Epithelial Cells”, Frontiers in Medecine, 2021
[5] J. S. H. Danial, Y. Aguib, M. H. Yacoub, “Advanced fluorescence microscopy techniques for the life sciences”, Global Cardiology Science & Practice, 2016
[6] R. Kaufmann, C. Hagen, K. Grünewald, “Fluorescence cryo-microscopy: current challenges and prospects”, Current Opinion in Chemical Biology, 2014
[7] G. Velve-Casquillas, J. Costa, F. Carlier-Grynkorn, A. Mayeux, P. T. Tran, “A Fast Microfluidic Temperature Control Device for Studying Microtubule Dynamics in Fission Yeast”, Methods in Cell Biology, 2010
[8] G. Velve-Casquillas, C. Fu, J. Cramer,S. Meance, A. Plecis, D. Baigl, J-J. Greffet, Y. Chen, M. Piel, P. T. Tran, “Fast microfluidic temperature control for high resolution live cell imaging”, Lab on a Chip, 2011
[9] F. Cantoni, G. Werr, L. Barbe, A. M. Porras, M. Tenje, A microfluidic chip carrier including temperature control and perfusion system for long-term cell imaging”, HarwareX, 2021
[10] F. Jiang, Y. Xu, J. Song, H. Lu, « Numerical Study on the Effect of Temperature on Droplet Formation inside the Microfluidic Chip”, Journal of Applied Fluid Mechanics, 2018
[11] B. Riechers, F. Wittbarcht, A. Hütten, T. Koop, “The homogeneous ice nucleation rate of water droplets produced in a microfluidic device and the role of temperature uncertainty” Physical Chemistry Chemical Physics, 2013
[12] D. Ross, L. E. Locascio, “Microfluidic Temperature Gradient Focusing”, Analytical Chemistry, 2002
[13] T. Matsui, J. Franzke, A. Manz, D. Janasek, “Temperature gradient focusing in a PDMS/glass hybrid microfluidic chip”, Electrophoresis, 2007
