PLGA Microparticles Synthesis
When PLGA is used as an active pharmaceutical ingredient carrier, it is important to produce highly monodispersed particles for drug release reproducibility. To reach this high monodispersity level, the RayDrop- developed and manufactured by Secoya- is used. The most common production process of PLGA microparticles is solvent based and can involve hazardous solutions. Ethyl acetate is preferred as it shows better biocompatibility than other conventional solvents such as dichloromethane.
Read the application note to learn how the PLGA microparticle production pack creates an excellent reproducibility and significantly improves monodispersity (CV < 2%) as compared to other methods on the market.
Introduction
Polymeric Microparticles (MPs) are popular carriers to increase the bioavailability and bio-distribution of both lipophilic and hydrophilic drugs (1).
What is PLGA ?
The Copolymer Poly(lactic-co-glycolic acid) (PLGA) is an FDA approved biocompatible material that is used for a variety of clinical and therapeutic applications ranging from intravenous and pulmonary drug delivery vehicles and anti-inflammatory implantable devices, to suture, scaffold, and graft materials. PLGA is degradable and bioabsorbable, making it highly desirable as a drug delivery device specifically in the form of micro and nanoparticles. In order to create PLGA microparticles as efficient drug delivery devices, it is critical to understand the physicochemical properties of PLGA as they help in the prediction and modification of polymer performance as it relates to drug stability and subsequent release (2).
PLGA degrades mainly via hydrolytic scission of the ester linkages between its lactic acid (LA) and glycolic acid (GA) moieties. The longevity of PLGA can be altered by changing the polymer length (molecular weight) and its end-capping—where another end group is substituted for the native carboxyl terminal groups (3).
Why use microfluidics for PLGA microbeads generation
Methods for making these particles have limitations and can be wasteful. The specific characteristics of the beads may differ greatly across the sample since conventional batch procedures generate particles and beads with a wide range of sizes in each batch. In contrast, microfluidic technologies allow for the single-step synthesis of highly monodisperse particles (2). Microfluidics, and more exactly droplet-based microfluidics, offer an efficient method for improvement. Droplet based microfluidics is a powerful tool which enables micrometric monodisperse droplet generation and manipulation. Typically, PLGA microparticle production involves emulsifying an organic droplet phase—which contains dissolved PLGA in solvent—in an aqueous continuous phase (containing an emulsion stabiliser), resulting in a polymer solution-in-water emulsion.
Currently, dichloromethane is the most common and widely utilized solvent in all microfluidic systems for the production of PLGA microbeads. However, this solvent has many limitations due to its toxicity. Because of its less harmful characteristics, ethyl acetate has been thought of as a substitute dispersed solvent suitable in synthesising polymer beads(4-5).
How to generate PLGA Microparticles using microfluidics
Materials
In this application note we will describe a novel platform and protocol to use ethyl acetate at different concentrations for PLGA polymerization and continuous synthesis.
Reagents
- Priming fluid: Ethyl acetate filtered with 0.2 μm pore filter and used without further modification (Sigma Aldrich, CAS Number: 141-78-6)
- Droplet fluid: Resomer® RG 753 S, Poly(D,L-lactide-co-glycolide) ester terminated, Lactide: Glycolide 75:25 is dissolved in ethyl acetate at room temperature by stirring over the course of an hour (Sigma aldrich,CAS Number: 26780-50-7).
- Continuous phase: 2 % (w/v) Poly (vinyl alcohol) (PVA) surfactant in water is filtered with a 0.2 μm pore filter (Sigma Aldrich,CAS Number: 9002-89-5).
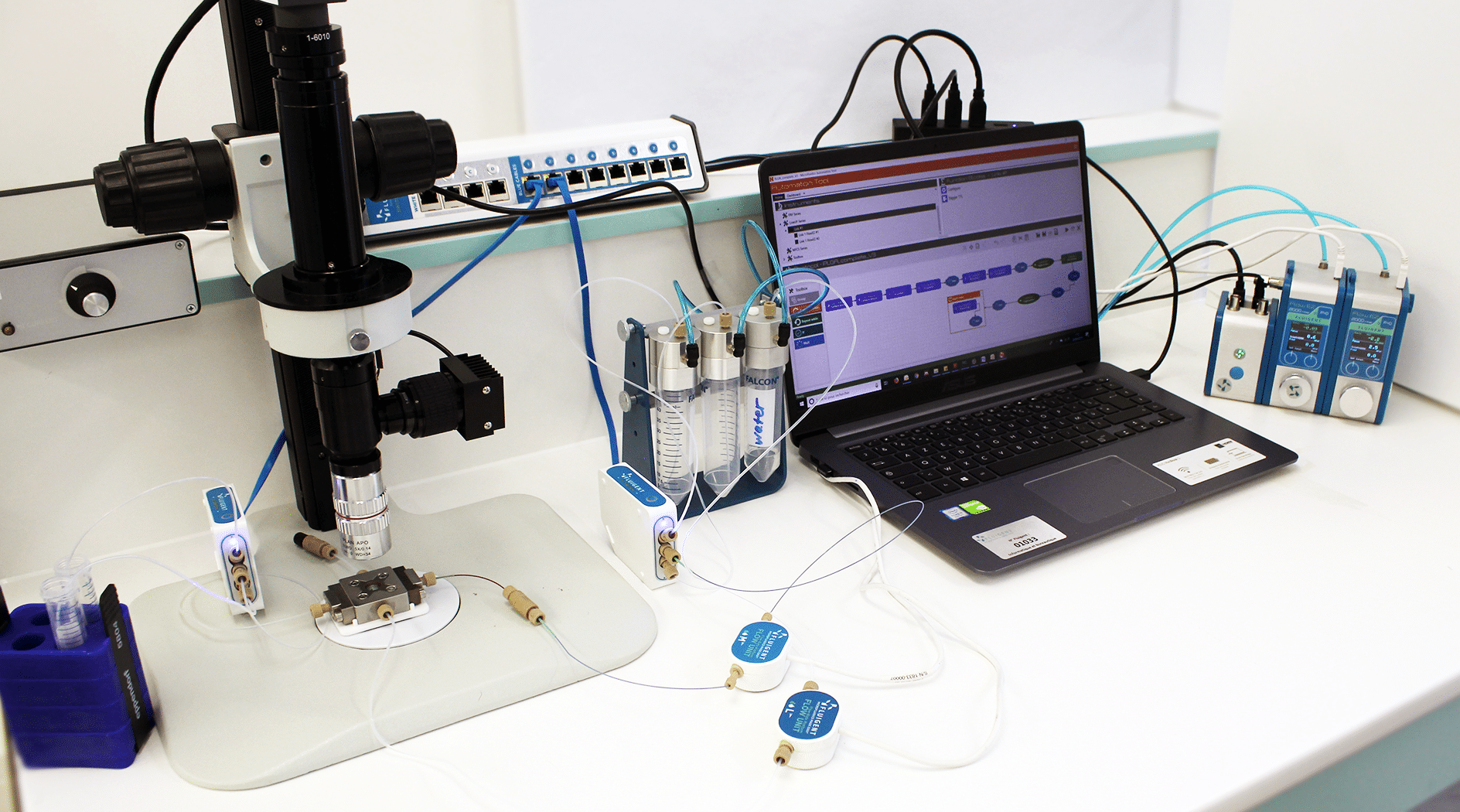
Methods: How to use droplet microfluidics for the generation of PLGA microbeads
Droplet Generation Experimental set-up
PLGA Microparticle Production Station
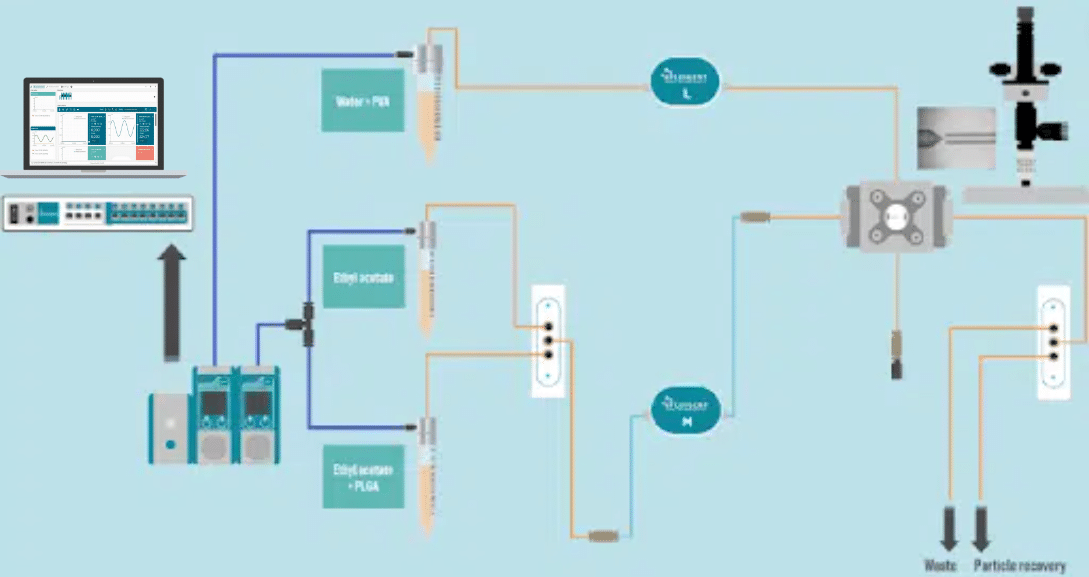
Two different pressure controllers (Flow EZTM, Fluigent are used to handle fluids. A 3/2 valve (2-SWITCHTM, Fluigent valve is used to switch between pure ethyl acetate and PLGA dissolved in ethyl acetate and a second valve is also used in particle production output to switch between a waste and recover sample.
Two FLOW UNIT sensors are used to monitor and control the internal and external phases flow rates during the run all.
The RayDrop Single Emulsion system is used to generate PLGA droplets.
Droplet Monitoring Analysis
An inverted microscope (Nikon, Eclipse Ti 2) and a high-speed camera (Nikon, PhotronFastcam) have been utilized to study droplet production.
The PhotronFastcam Viewer program has been used to analyze droplet sizes.
PLGA Microparticle Precipitation
When droplets are formed, the solvent inside the microparticles constantly diffuses out of the droplet while the continuous phase is present.
The concentration of PLGA will increase with solvent diffusion outside the droplet and precipitate to create a solid polymer beads that is smaller than the droplet.
PLGA Microparticle Recovery
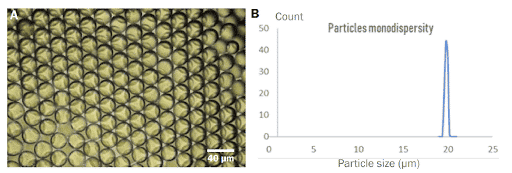
PLGA Microparticles are recovered in a vial containing the continuous phase: water with 2% PVA. The microparticle size and monodispersity are determined under a microscope.
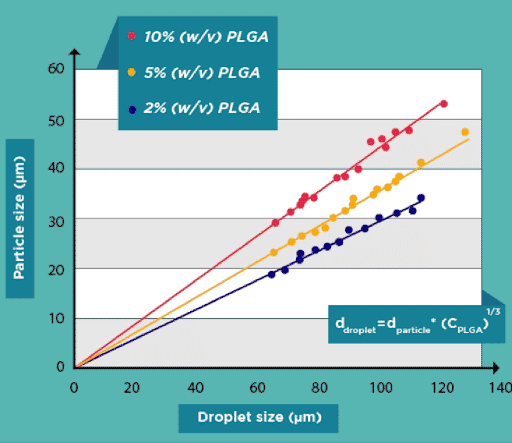
Results
Polymerization of microfluidics-produced liquid crystal double emulsions for making wavelength and polarization-selective retroreflectors
In this webinar by the Experimental Soft Matter Physics (ESMP) group at the University of Luxembourg in collaboration with Fluigent, we will demonstrate, first, that the liquid crystal shells form a rich and intriguing platform for innovative photonics research.
✅ Be introduced to the amazing photonics of cholesteric liquid crystals and how their self-assembling behavior aligns perfectly with microfluidics;
✅ Learn about the interesting and useful consequences of photopolymerizing asymmetric shells around an incompressible liquid core;
✅ Be inspired by the diverse opportunities to apply the solid spheres created by polymerizing cholesteric liquid crystal shells;
✅ Find that all equipment and materials required to make these peculiar selective retroreflectors are commercially available, making it very easy to get started making your own cholesteric spherical retroreflectors.
Conclusion
Successful production of PLGA microbeads with diameters between 15 and 50 µm has been accomplished. In comparison to other technologies available on the market, the PLGA microparticle production pack enables great reproducibility and significantly increased monodispersity (CV 2%). It enables uninterrupted, long-term production of PLGA microparticles for use in investigations.
A PLGA microparticle synthesis package has been created by Fluigent to enable high-quality, continuous production for common investigations. It brings together all the benefits of pressure-based solutions and droplet microfluidics to focus on the experiment, and even offers the chance to automate protocols for producing different microparticle sizes.
Related Resources
Drug encapsulation in biocompatible microparticles for drug delivery
DiscoverWEBINAR – Raydrop, a universal droplet generator based on a non-embedded co-flow-focusing
Discover- 기술서
Droplet-based Microfluidics
Discover - Support & Tools
Droplet Size Calculator
Discover - 어플리케이션 노트
Droplet Sequencing: Drop-Seq method
Discover
REFERENCES
- Lagreca, E., Onesto, V., Di Natale, C., La Manna, S., Netti, P., & Vecchione, R. (2020). Recent advances in the formulation of PLGA microparticles for controlled drug delivery. Progress In Biomaterials, 9(4), 153-174. doi: 10.1007/s40204-020-00139-y
- Rapier, C., Shea, K., & Lee, A. (2021). Investigating PLGA microparticle swelling behavior reveals an interplay of expansive intermolecular forces. Scientific Reports, 11(1). doi: 10.1038/s41598-021-93785-6
- Keles, H., Naylor, A., Clegg, F., & Sammon, C. (2015). Investigation of factors influencing the hydrolytic degradation of single PLGA microparticles. Polymer Degradation And Stability, 119, 228-241. doi: 10.1016/j.polymdegradstab.2015.04.025
- Cho, M., & Sah, H. (2005). Formulation and process parameters affecting protein encapsulation into PLGA microspheres during ethyl acetate-based microencapsulation process. Journal Of Microencapsulation, 22(1), 1-12. doi: 10.1080/02652040400026269
- Sani, S., Das, N., & Das, S. (2009). Effect of microfluidization parameters on the physical properties of PEG-PLGA nanoparticles prepared using high pressure microfluidization. Journal Of Microencapsulation, 26(6), 556-561. doi: 10.1080/02652040802500655