E. Coli Culture in Droplets Using dSURF Fluorosurfactant
In-vitro cell culture is a fundamental component of biological production systems and biotechnological research. The ability to grow cells outside of their natural environment offers many possibilities ranging from high quantity production of enzymes to cell toxicity studies.
This application note was made in collaboration with Oksana Shvydkiv and her lab to highlight the biocompatibility of the surfactant dSurf by performing an E. Coli culture in droplets.
Introduction to E. Coli Culture in Droplets
Traditional and new methods for cell culture
Petri plates or culture flasks have traditionally been used in laboratory cell cultivation as part of in-vitro procedures. In culture flasks, cells are grown in a homogenous liquid medium, while Petri dishes allow the growth of colonies on a solid or semi-solid substrate surface. However, these two approaches have limitations, such as the challenge of compartmentalizing clones and single cells.
To overcome these limitations related to culture in Petri dishes or culture flasks, droplet culture of microorganisms, which allows homogeneous growth of cells, is proposed as an alternative method. In this case, E. Coli culture in droplets will be performed. The ability to miniaturize microscale droplets offers great advantages due to their higher surface-to-volume ratio. This feature confers faster mixing and heat transfer, which accelerates reaction times. In addition, droplets are isolated monodisperse chambers that act as reproducible microreactors that can be created with high throughput.
Microfluidics to ensure good monodispersity and stability
However, to be able to use droplets for pharmaceutical and biomedical applications, it is necessary to achieve and guarantee a high degree of monodispersity and stability with such a method. Typically, single emulsions are produced in batches by a two-stage emulsification process (mixing in bulk), resulting in a highly polydisperse population with low encapsulation efficiency.
The use of microfluidics for droplet generation can be a very useful tool to overcome the problems of these conventional methods. In microfluidics, highly reproducible, continuous and reproducible droplet production requires an efficient microchip , a stable fluid handling system (consisting of pulseless pressure-based controllers) and an adapted surfactant.
Why use surfactants in droplet-based microfluidics ?
Surfactants are an essential part of droplet-based microfluidic technology. They are involved in the stabilization of droplet interfaces, in the biocompatibility of the system, and in the process of molecular exchange between droplets. Typically, on encapsulation mechanisms, mineral oils have been the most commonly used with cells. They are therefore limited to the applications such as PCR where the objects of interest (the DNA or RNA fragments) do not exchange between the droplets.
For E. Coli culture in droplets, perfluorinated oils have shown several advantages compared to other carrier fluids such as mineral oils. Their low viscosity allows easy handling in microfluidic systems without need of high-pressure pumps. Though perfluorinated oils have advantages for applications like cell culture and dPCR, it is still difficult to develop a suitable and effective surfactant for droplet stabilization in these oils. To date, most commercially available surfactants present limitations.
The objective of this study is to highlight the biocompatibility of the new surfactant dSURF by performing E. Coli culture in droplets using Fluigent’s pumping technology.
How to perform an E. coli culture
Reagents:
Continuous phase Reagents:
Novec HFE-7500 (Sigma Aldrich) containing 0.5% or 3% dSURF
Dispersed phase Reagents:
500µl suspension of E. coli ECJW922 in TB (Terrific broth) medium with OD600 (optical density) of 0.005 (5 x 106 CFU/ml) or 0.01 (10 x 107 CFU/ml), which means cell concentration in the first suspension was 5 x 106 CFU/ml and in second suspension 10 x 107 CFU/ml. CFU – colony forming unit.
How to generate droplet for encapsulation purpose
E. Coli culture in droplets was performed with customer designed PDMS chip, but we would recommend using the EZ drop chip.
To generate droplets, fluid-handling devices such as pressure controller, syringe pump, or peristaltic pump can be used with a flow-focusing PDMS chip.
Pressure controllers such as Fluigent LineUPTM series or MFCSTM-EZ are best suited to optimize droplet generation performance, improving Flow rate stability and droplet monodispersity. In this case, E Coli suspension and dOIL with dSURF are loaded into vials.
A pressure is applied to the reservoir to ensure a continuous and pulseless injection of both phases into the chip. The determined flow rate is monitored and controled by using OxyGEN software or local control on the Flow EZTM to achieve the desired droplet size and frequency.
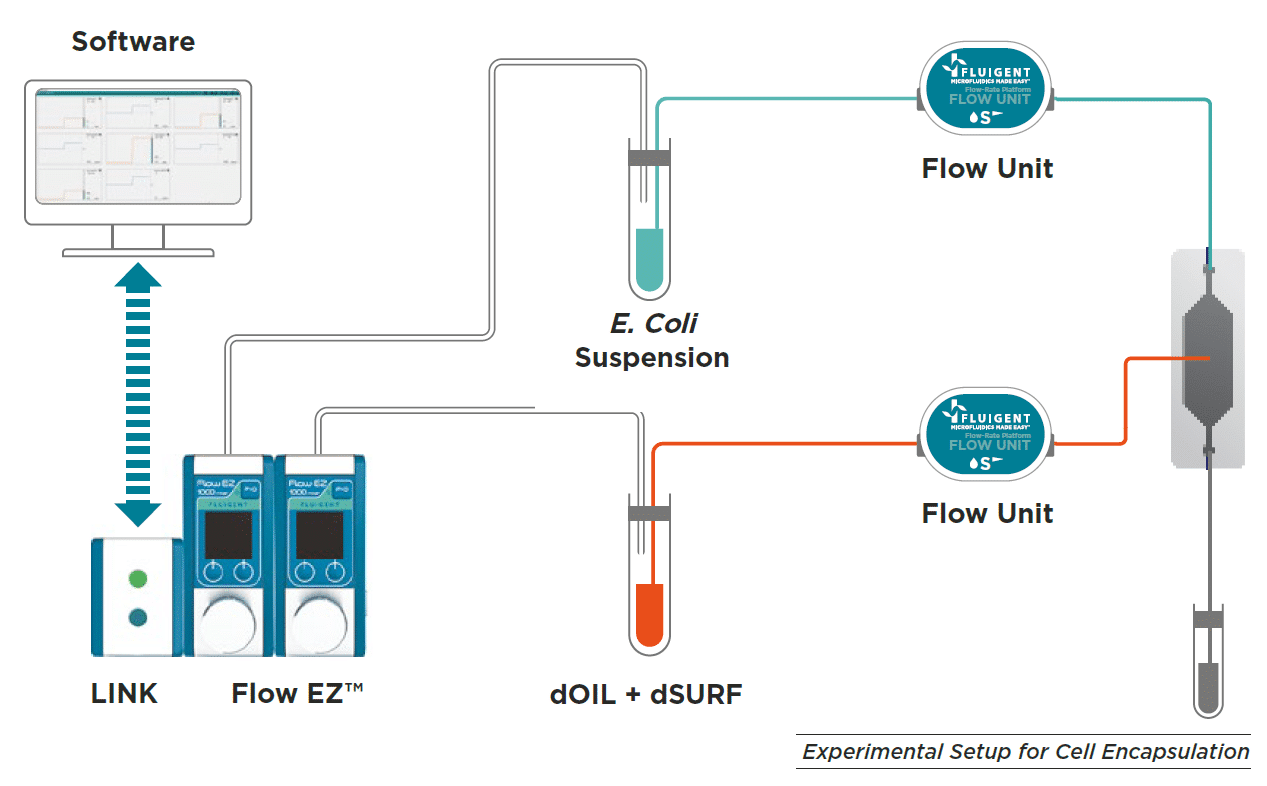
Material for Escherichia coli culture in droplets
Observation of the droplets produced
Test with E. coli cells at OD= 0.005 and 0.5% surfactant concentration, incubation at 37 °C:
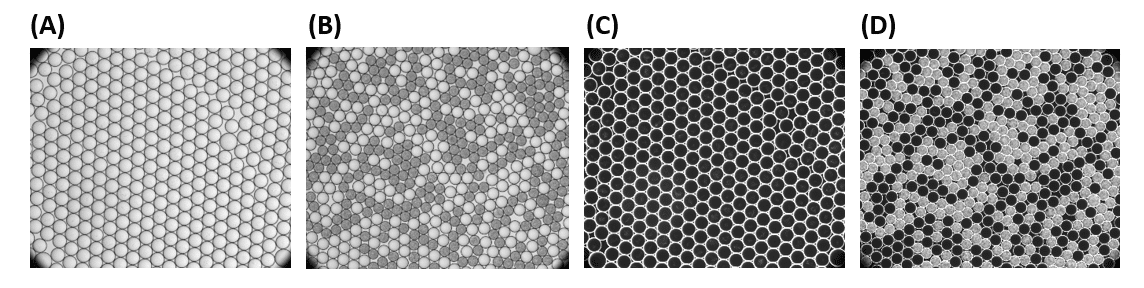
C) Images of droplets under dark-field after 4 h incubation (D) Images of droplets under dark-field after 20 h incubatio
Stable, monodispersed droplet emulsions were observed on both images. High numbers of E. coli cells were observed in almost half of the droplets, particularly in 46±0.8 % of droplets, which is in a good agreement with the theoretical number of 54% calculated from the Poisson distribution of cells over 33.6 µm droplets (160 pL) at a given concentration of 5 x 106 CFU/ml.
The choice to use a low E. coli concentration and fractional droplet occupation with cells, as well as small variations in the experimental process, such as general pipetting imprecision, may help to explain the disparity between theoretical and experimental numbers.
Conclusion
The growth of the E. Coli culture in droplets after 20 h of incubation in all above-described cases has highlighted that dSURF is biocompatible at concentrations ranging from 0.5% to 3%. For routine applications and cost reduction, 0.5% concentration of dSURF can be used. It is also suitable for experiments starting with single and multiple microbial cells per droplet.
The emulsion stability after 20 h shows the good droplet stabilization which will be of use for longer duration experiments.This study has been made in collaboration with Dr Oksana Shvydkiv and her lab from Leibniz Institute for Natural Product Research and Infection Biology.




