Emulating the chondrocyte microenvironment using multi-directional mechanical stimulation in a cartilage-on-chip
Paggi et al. have implemented a cartilage-on-chip platform which can generate both compressive and multi-directional mechanical stimulations on chondrocyte-laden hydrogels and join modelling.
Hence, chondrocytes can experience healthy and/or hyper-physiological loading as the joint would do during movement depending on the amount of applied load. Collectively, their results highlight the importance of applying proper mechanical stimulation for proper regulation of chondrocytes and reproducing their microenvironment through the production of native pericellular matrix.
Carlo Alberto Paggi, Jan Hendricks, Marcel Karperien and Séverine Le Gac
Lab on Chip, 2022, 22 (DOI: 10.1039/d1lc01069g)
Introduction
Mechanical stimulation of chondrocytes plays essential roles in the homeostasis of cartilages and arthritic conditions. The knee, devoid of conventional transport systems like blood vessels and nerves, relies on mechanical stimulation as the principal mediator for intercellular communication. Within this unique environment, signaling molecules and nutrients navigate through load-induced extracellular fluid flow.
Layers of Defense: Interstitial and Pericellular Matrices
The cartilage structure is predominantly composed of the interstitial matrix, adept at absorbing compressive and sliding forces encountered during movement. Chondrocytes, nestled within a softer pericellular matrix, find protection from external forces exerted on the interstitial layer. This two-layered defense mechanism allows chondrocytes to sense extracellular matrix deformation during joint movement.
Organ-on-Chip Models with Mechanical Ingenuity
To explore the impact of mechanical stimulation, organ-on-chip join models have been developed incorporating a mechanical actuation unit.
Paggi et al. introduce a cutting-edge cartilage-on-chip platform capable of orchestrating both compressive and multi-directional mechanical stimulations on chondrocyte-laden hydrogels. This innovative setup mimics the diverse loading scenarios experienced by chondrocytes during joint movement.
In their groundbreaking study, Paggi et al. shed light on the paramount importance of applying precise mechanical stimulation
The research not only emphasizes the nuanced regulation of chondrocytes under different loading conditions but also stresses the need to faithfully replicate the native microenvironment. The production of a native pericellular matrix emerges as a key factor in faithfully reproducing the intricate tapestry of cartilage physiology.
This exploration into the world of mechanical stimulation and its profound impact on cartilage dynamics paves the way for a deeper understanding of joint health and opens avenues for innovative therapeutic interventions.
Experimental procedure
The platform is composed of a central cell culture chamber caught in between a perfusion channel and a mechanical actuation unit. The actuation unit is made of a 50µm thick PDMS membrane actuated by three individual chambers connected to each other.
Cells are encapsulated within an agarose hydrogel in the central chamber and can be mechanically stimulated by the deflection of the PDMS membrane. Pressure is generated using Fluigent positive pressure controller MFCS-EZ. When the same positive pressure is applied in the three actuation chambers, the compression is homogeneous.
Different deformations patterns can be obtained using a dedicated sequence of positive and negative pressures in the different actuation chambers, giving rise to a multi-directional mechanical stimulation (mdms).
This mdms pattern was shown to consist of a combination of compressive and shear forces, and is reminiscent of a waveform of mechanical stimulation, mimicking the rolling motion of two cartilage surfaces.
In this figure, the cartilage-on-chip design is described. It is compared to a knee joint (a)) and the main features of the device are higlighted in b). In c) picture food dyes are used for visualization purposes: the actuation unit is in blue, the cell-hydrogel chamber is in red and the perfusion channel is in yellow.
Finally, in d) and e), multi-directional mechanical stimulations are depicted for a knee joint during motion and fort the organ-on-chip join device filled in with human chondrocytes in agarose (blue arrows depict compression and green arrows shear strain).
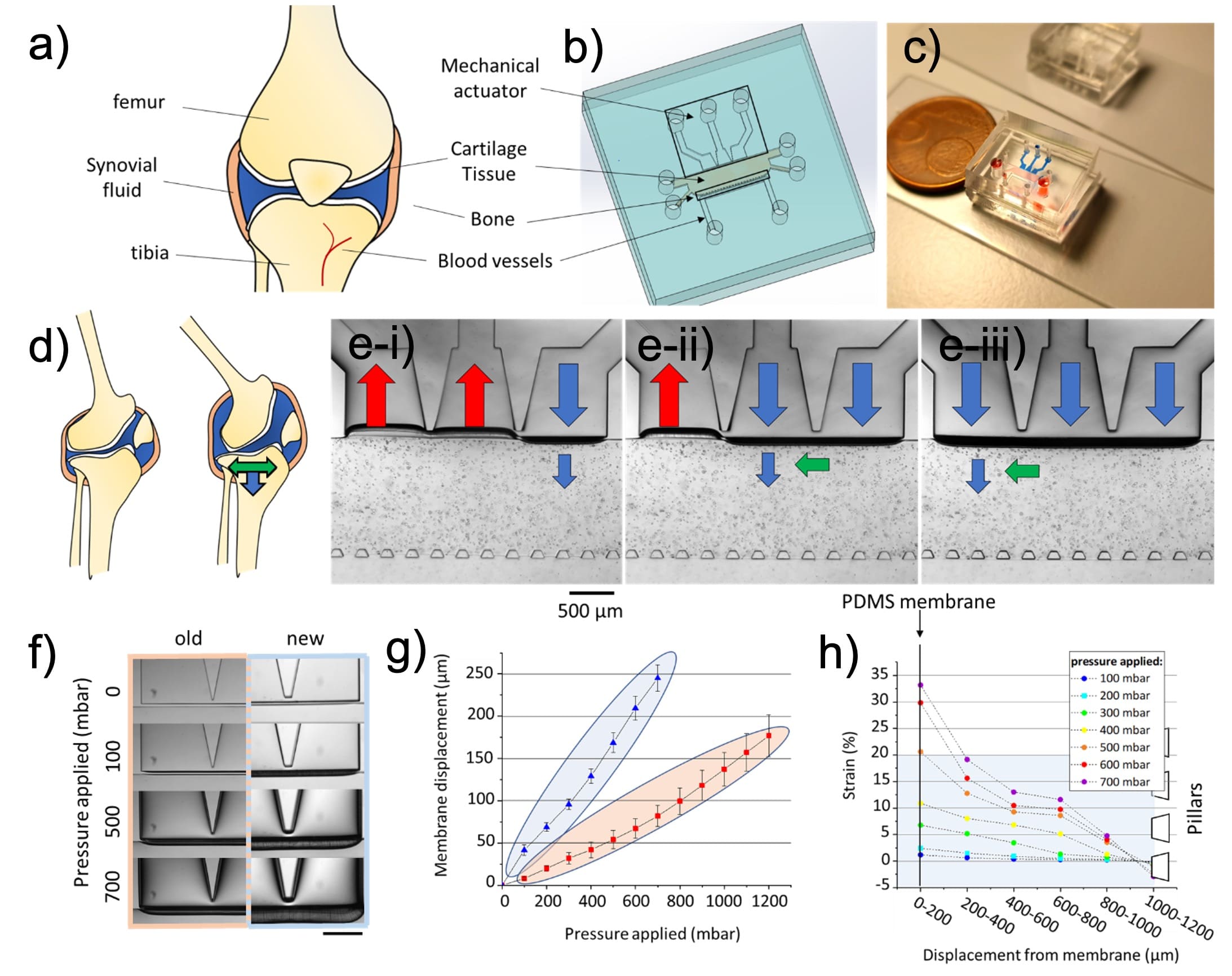
Cartilage-on-chip design (from Paggi et al. 2022)
Results
Chondrocytes embedded in agarose hydrogel within the device displayed a pro-inflammatory response (cytokine release) which was further amplified by mechanical actuation. Exposure to mechanical stimulation and especially to an mdms pattern was found to have an impact on gene expression of chondrocytes markers.
Remarkably, the production of glycosaminoglycans (GAGs), one of the main components of native cartilage ECM, was significantly increased after 15 days of on-chip culture and 14 days of mechanical stimulation.
A thin pericellular matrix shell (1–5 μm) surrounding the chondrocytes as well as an interstitial matrix, both reminiscent of the in vivo situation, were deposited. Matrix deposition was highest in chips exposed to mdms stimulation.
Finally, mechanical cues enhanced the production of essential cartilage ECM markers, such as aggrecan, collagen II and collagen VI, a marker for the pericellular matrix around the chondrocytes.
Both mechanical stimulation options promote the production of extracellular matrix by the chondrocytes.
Pericellular matrix production around each individual cell was comparable for both mechanical stimulation strategies (indicated by the blue ring shown on histology sections).
However, MDMS was observed to significantly enhance the production and distribution of the interstitial matrix throughout the entire tissue, as evidenced by the consistent blue coloration.
Mechanical stimulation of the chondrocytes thereby allows emulating the native articular cartilage microenvironment, with a clear advantage of mdms stimuli.
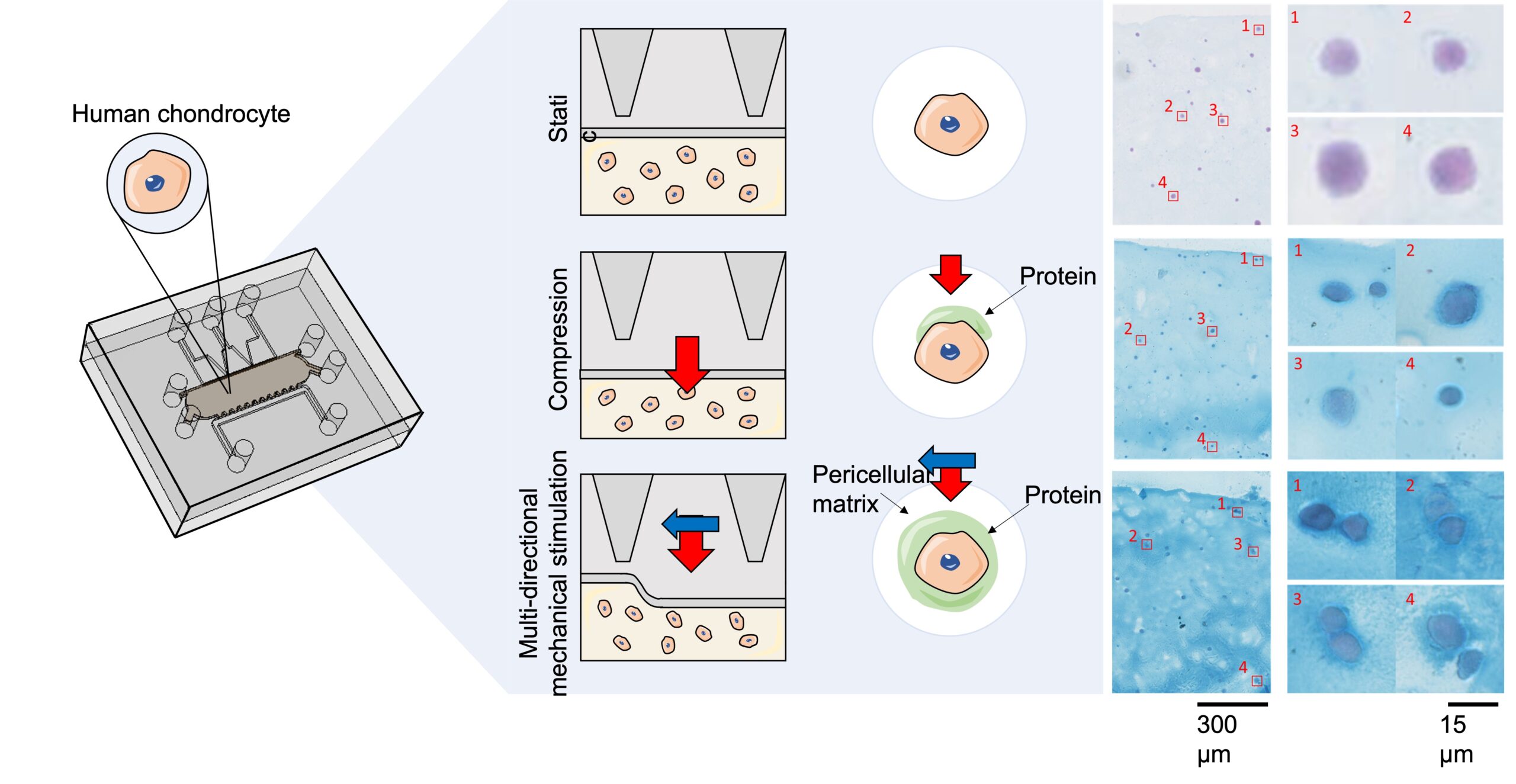
Effect of mechanical stimulation (compressive forces only or multi-directional mechanical stimulation) on chondrocyte functions within the platform
Conclusion
Using a custom-built cartilage-on-chip platform coupled to Fluigent MFCS pressure controllers which provide excellent control on the applied pressure, immediate switching time response and offers a user-friendly and programmable interface, the authors were able to accurately apply a variety of mechanical forces on cells embedded within an agarose hydrogel.
They have demonstrated how mechanical stimulation of the chondrocytes can help to reproduce the native articular cartilage microenvironment, with an ever more pronounced benefit of multi-directional mechanical stimuli than homogeneous compression, in their platform. Altogether this research article highlights the importance of imposing appropriate mechanical cues to emulate in vitro the chondrocyte microenvironment.

“For our application, where we include mechanical stimulation in organ-on-chip models, we love all Fluigent equipment which provides us full flexibility, fast response time, and user-friendliness. Support has been amazing as well to optimize and customize set-ups.”
Dr Séverine Le Gac – Associate Professor & Head of Applied Microfluidics for BioEngineering Research (AMBER) – University of Twente (The Netherlands)
Related Products
Related Resources
- 어플리케이션 노트
Peristaltic Pump vs Pressure-Based Microfluidic Flow Control for Organ on Chip applications
Discover - 압력 기반 미세유체 제품의 이점
Microfluidic pressure control for organ-on-a-chip applications: A comprehensive guide
Discover - 어플리케이션 노트
Long-term fluid recirculation system for Organ-on-a-Chip applications
Discover 
Webinar – Organ on a Chip Towards the Next Generation of Cell Culture Platforms
Discover



