Microbubble formation using the RayDrop
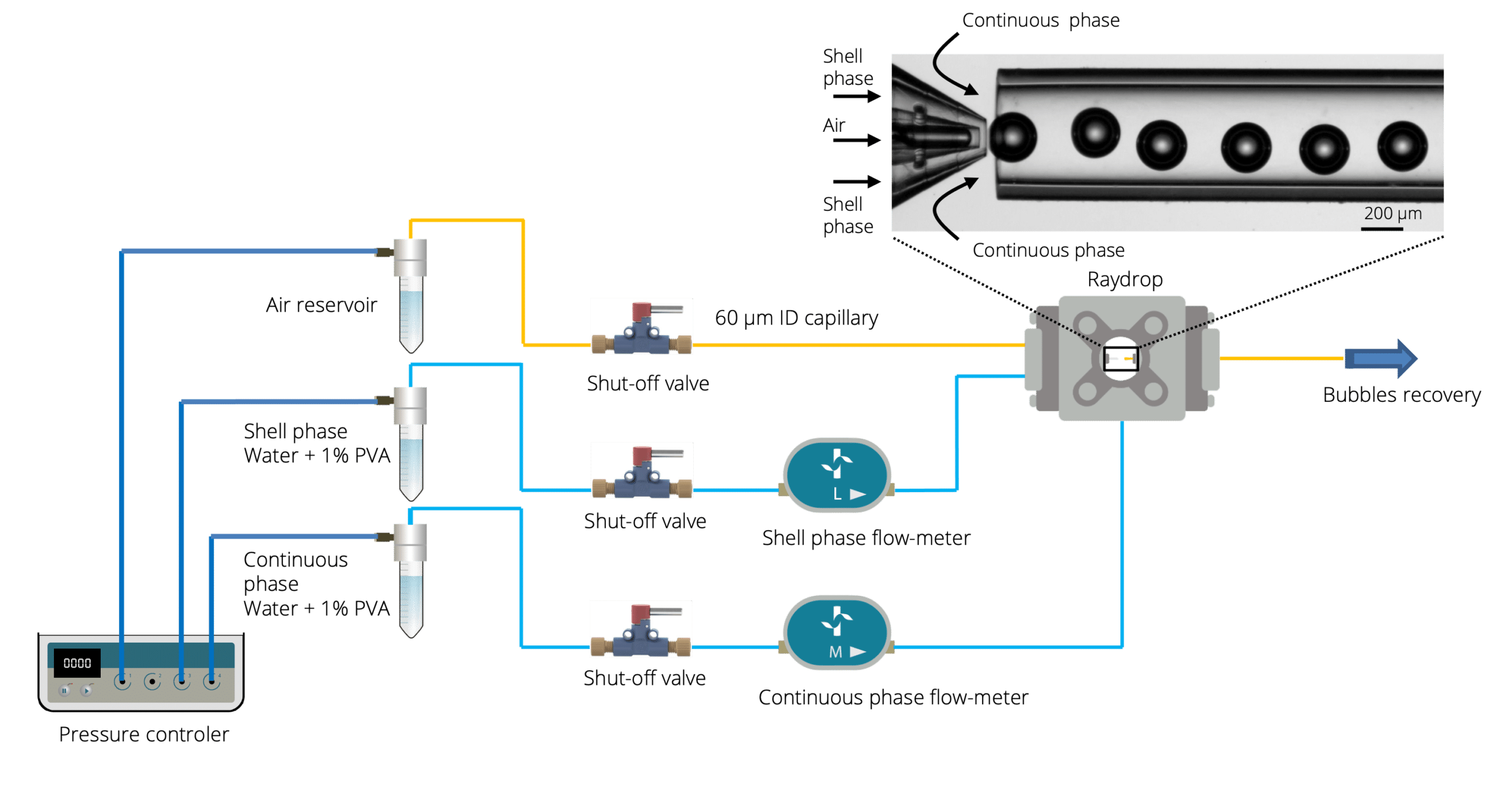
Microbubbles were generated using the RayDrop Double Emulsion developed and manufactured by Secoya, a capillary-based microfluidic device equipped with a 3D printed injection nozzle in combination with pressure-based flow controllers. We investigated how parameters, such as the geometry of the nozzle and the continuous-phase flow rate, affect the microbubble formation process.
Introduction to Microbubble Generation
What are the applications of microbubble formation?
The microfluidic community is increasingly exploring microbubble formation due to its potential applications across various fields, including industry, life sciences, medicine, and material sciences (1).
The controlled generation of microbubbles in microfluidic devices holds significant promise in medicine, enabling non-invasive imaging of molecular events using targeted microbubbles. In the future, imaging methods are expected to be commonly employed to define pathophysiology and develop and test new therapeutic approaches for conditions such as inflammatory disorders, cancer, and cardiovascular disease (2).
Micron-sized bubbles find fundamental medical applications, serving as ultrasound contrast agents, contributing to thrombus destruction, facilitating targeted drug delivery, enabling tumor destruction, and functioning as a flotation column. Moreover, intravenous injection of a stabilized solution containing sufficiently small bubbles may be considered for acute lung dysfunction (4). Microbubble generation also plays a role in studying gas-liquid physical processes, including the dissolution of CO2 in solvents, CO2 reaction, and sequestration. This concept extends to foams, which are formed by trapping pockets of gas in a liquid or solid (5).
How to Remove Bubbles from Microfluidics
The occurrence of air bubbles in a liquid mirrors the process of liquid or oil droplet formation. This phenomenon initiates with the elongation of the flowing substance—be it oil, water, or air—culminating in the thinning and eventual pinch-off of the “neck” connecting the droplet or bubble to the flowing material. This pinch-off event facilitates the collapse of the droplet or bubble into a spherical shape.
In the realm of microbubble formation, precise control over size and distribution is paramount for various applications. Achieving monodisperse microbubbles is crucial for fundamental studies, simplifying the interpretation of experimental results compared to polydispersed counterparts. Monodisperse microbubbles also serve as valuable systems for measuring essential properties (3, 4).
Explore effective methods to eliminate bubbles and optimize microfluidic processes for enhanced results.
What is the best method to generate microbubble?
The best production mode to obtain stable and monodispersed droplets and/or microbubbles follows the dripping regime, with the droplet/bubble detaching from the jet at the junction between the two immiscible phases.
Therefore, in this application note, we demonstrate that a stable and continuous formation of microbubbles can be achieved using both RayDrop Single Emulsion and RayDrop Double Emulsion technologies.
In addition, we also studied in depth the parameters that affect microbubble production, such as nozzle geometry and continuous phase flow rate.
We found that, when using RayDrop Single Emulsion, the low-viscosity continuous phase has little impact on microbubble size formation. Consequently, in this case, the microbubble size is a function of capillary size. Therefore, the nozzle geometry only influences the microbubble size when decoupled from the continuous phase flow.
On the other hand, we found that, using RayDrop Double Emulsion technology, the production of microbubbles with tunable size and a polydispersity index value of less than 1% is possible.
We conclude, therefore, that the process of microbubble formation using the double emulsion RayDrop technique seems to be more suitable for controlling the size and monodispersity of microbubbles.
How to form microbubble
Microbubble formation methods
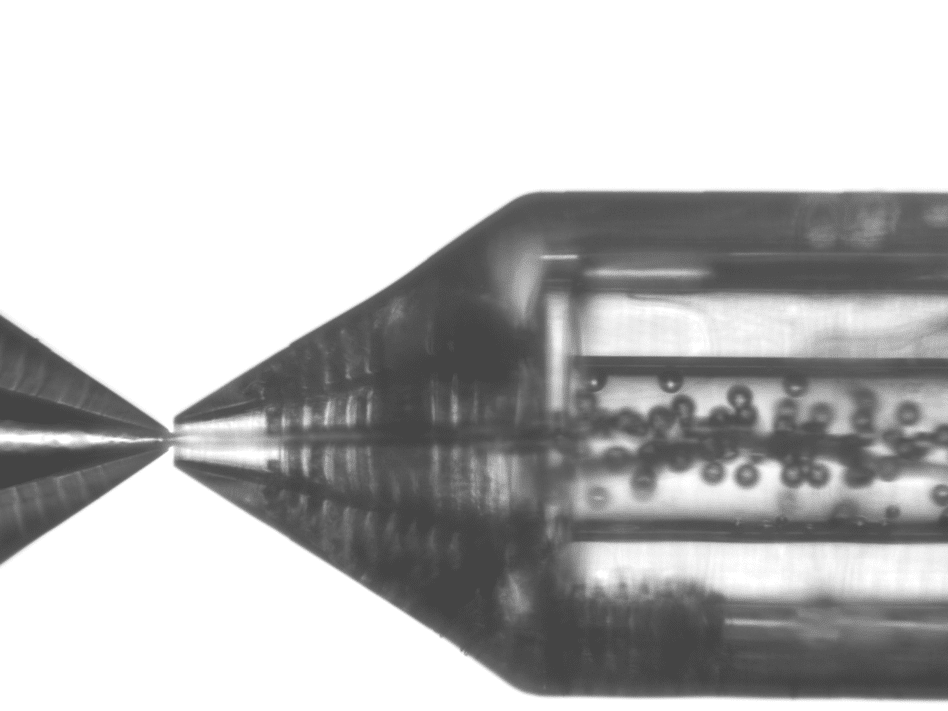
Microbubbles were created using the RayDrop Double Emulsion by injecting air (core phase) into an aqueous shell phase before being further engulfed by the continuous phase (Figure 1). A 1% solution of polyvinyl alcohol constitutes the liquid phases of the shell and continuous phases. The three components were pumped using a pressure-based controller to create microbubbles. Flowmeters were used to regulate the flow rates.
The different geometries of the Secoya’s chip
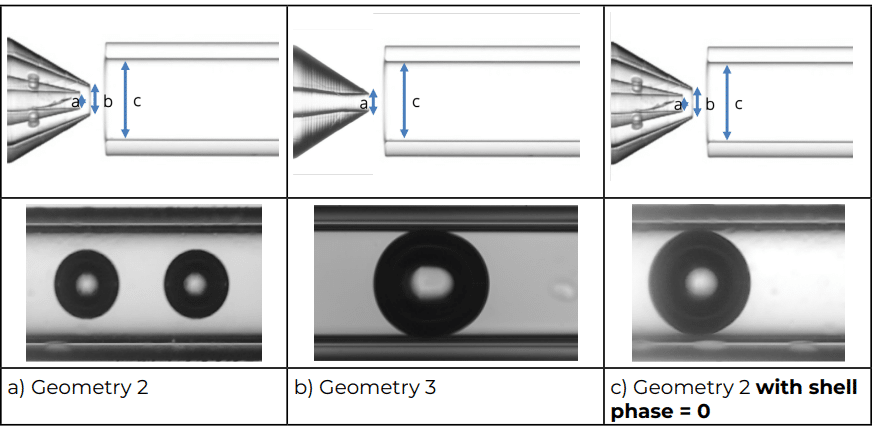
Three different geometries were used in this note (Figure 2) to assess the advantages of using double emulsion Raydrop (Secoya Technologies) for accurate microbubble size control.
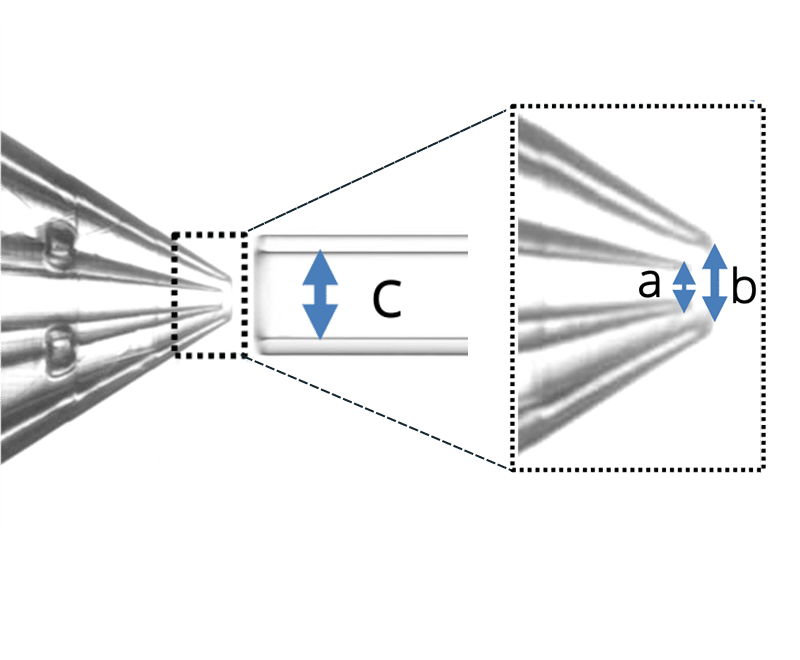
Geometry 1:
- a = 30 µm
- b = 70 µm
- c = 150 µm
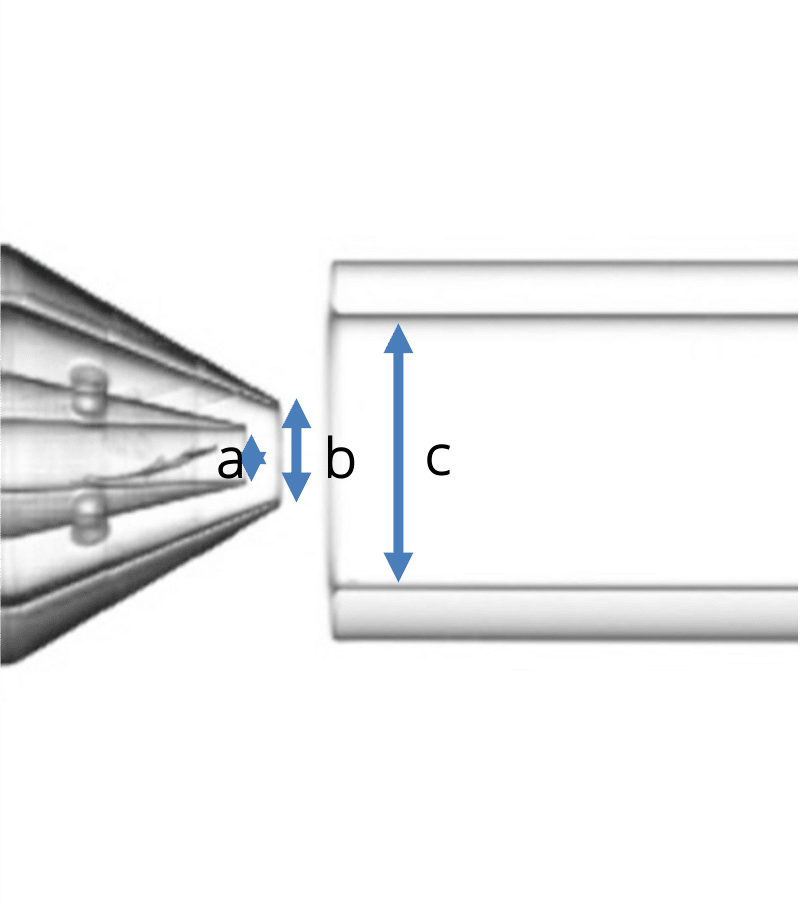
Geometry 2:
- a = 90 µm
- b = 160 µm
- c = 450 µm
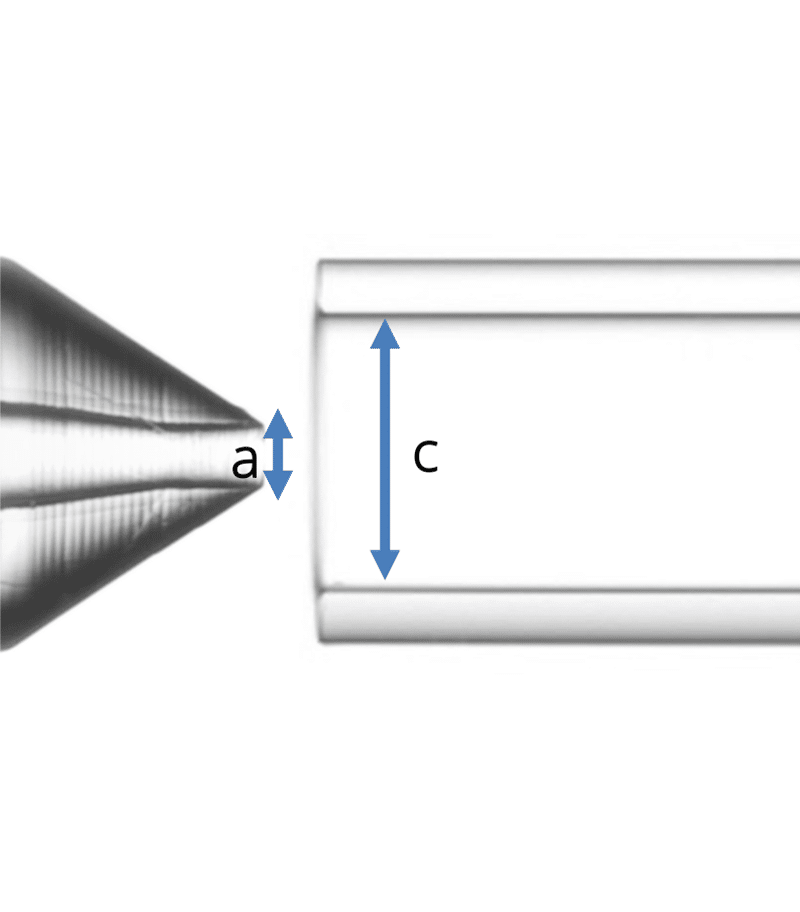
Geometry 3:
- a = 90 µm
- c = 450 µm
Figure 2: The three geometries used in the study
Results of Microbubble Formation
Geometry 3 and Geometry 2 were chosen to compare the simple emulsion nozzle with the double emulsion nozzle to produce microbubbles and results are presented in Figure 3. This clearly shows the influence of the shell stream on the size of the bubbles.
b) Images from human skin (five cycles, 15 of 19 parameters shown). Scale bars: 200 µm (left), 25 µm (insets). Keratin 10 (K10), Keratin 14 (K14).
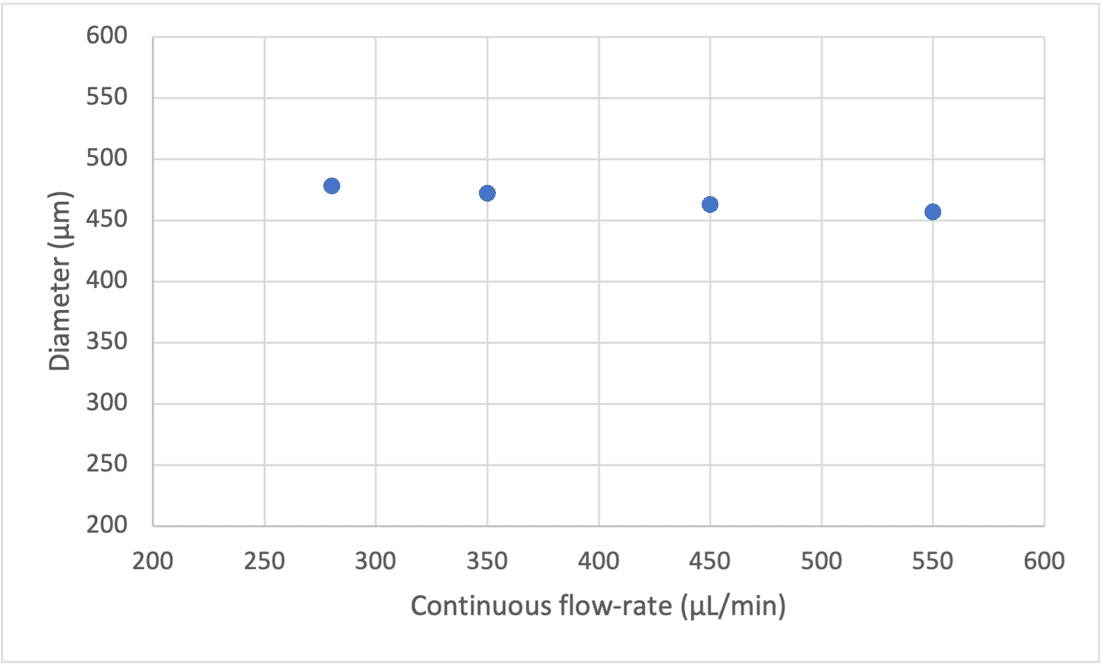
Using single emulsion nozzle (Geometry 3) and a low viscosity liquid for microbubble formation, the size of the microbubbles is almost totally constrained by the geometrical parameters the lowest size will be limited by the diameter of the extraction capillary (Figure 4).
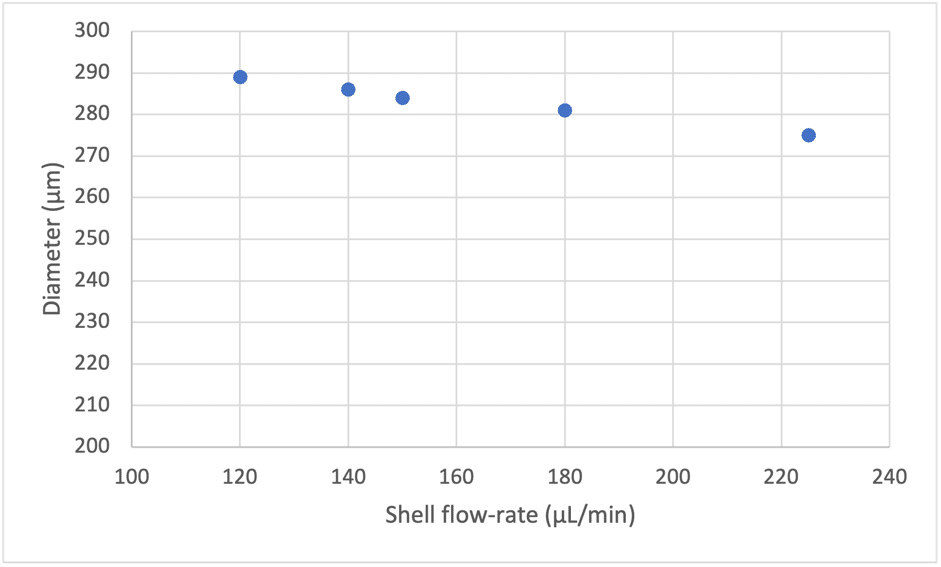
With the double emulsion nozzle (Geometry 1 and 2), the lowest size achievable is determined by both the geometrical parameters and the stream of the shell phase (Figure 5). The presence of the shell phase stream shifts the achievable size range towards lower values.
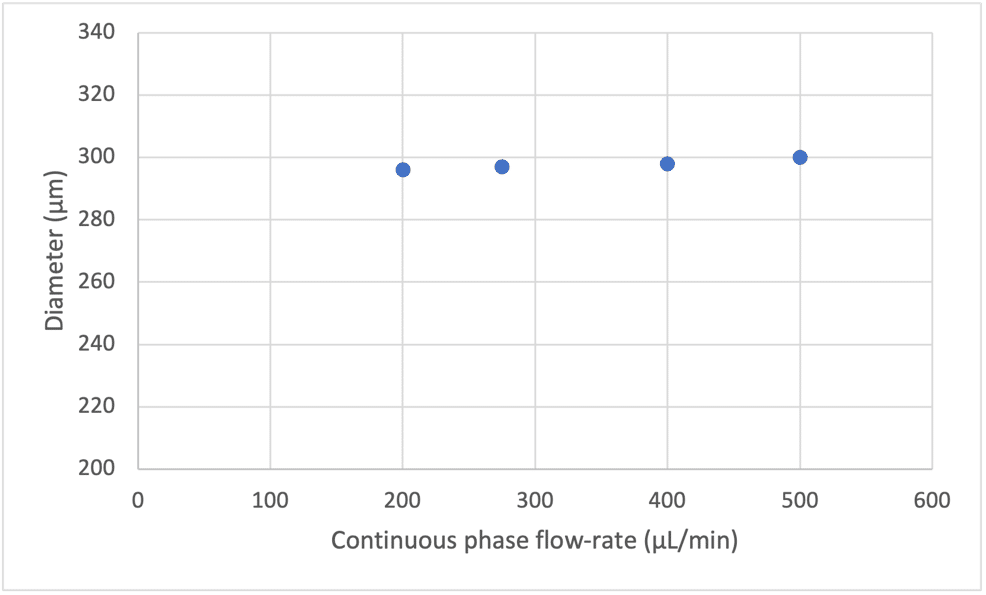
We can observe that with constant flow rates in the shell and core using a double emulsion nozzle, the increase of the continuous phase has no effect on the bubble size (see Figure 6).
The geometry of the nozzle-capillary system is the most influential factor on bubble size. To optimize the system one should make the inside diameter of the capillary equivalent to the targeted bubble size. Local restriction can be obtained by using a second nozzle attached on the entry of the extraction capillary (Figure 9). With this specific geometry, air bubbles of 30 µm diameter were generated in water at hundreds kHz.
Download the application note for more results.
Conclusion
In the last decade, microfluidic technology has emerged as one of the most efficient methods for microbubble formation, since it allows the generation of highly monodisperse droplets through the reproducibility of the process.
These uniform microbubbles are good items for the basic research of mass-transfer and chemical reaction processes at the micrometer scale. For the controllable application of microbubbles, the microfluidic generation mechanism used is very important.
We here show that the production of microbubbles using the double emulsion RayDrop technique is well-suited to control the size and the monodispersity of microbubbles. Of note, the geometry of the nozzle only impacts the size of the bubble while it is decoupled from the continuous-phase flow rate.
Expertises & Resources
References
- Wan, J. et al. (2008) “Controllable microfluidic production of microbubbles in water-in-oil emulsions and the formation of porous microparticles,” Advanced Materials, 20(17), pp. 3314–3318. Available at: https://doi.org/10.1002/adma.200800628.
- Lindner, J.R. (2004) “Microbubbles in medical imaging: Current applications and Future Directions,” Nature Reviews Drug Discovery, 3(6), pp. 527–533. Available at: https://doi.org/10.1038/nrd1417.
- Wang, K. et al. (2013) “Generating microbubbles in a co-flowing microfluidic device,” Chemical Engineering Science, 100, pp. 486–495. Available at: https://doi.org/10.1016/j.ces.2013.02.021.
- Xu, J.H. et al. (2006) “Formation of monodisperse microbubbles in a microfluidic device,” AIChE Journal, 52(6), pp. 2254–2259. Available at: https://doi.org/10.1002/aic.10824.
- Parmigiani, A. et al. (2016) “Bubble accumulation and its role in the evolution of magma reservoirs in the Upper Crust,” Nature, 532(7600), pp. 492–495. Available at: https://doi.org/10.1038/nature17401.