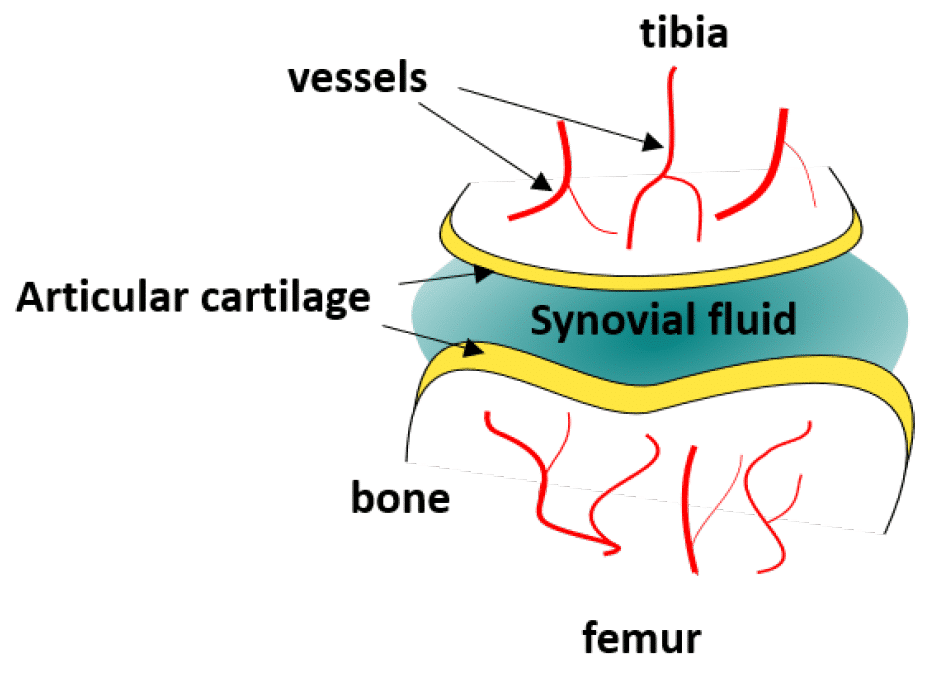
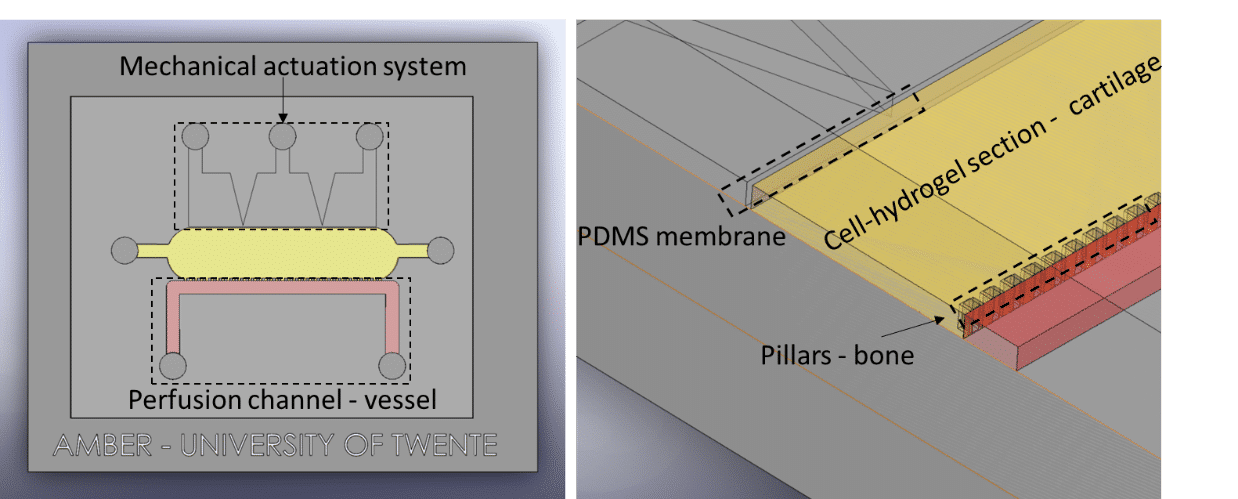
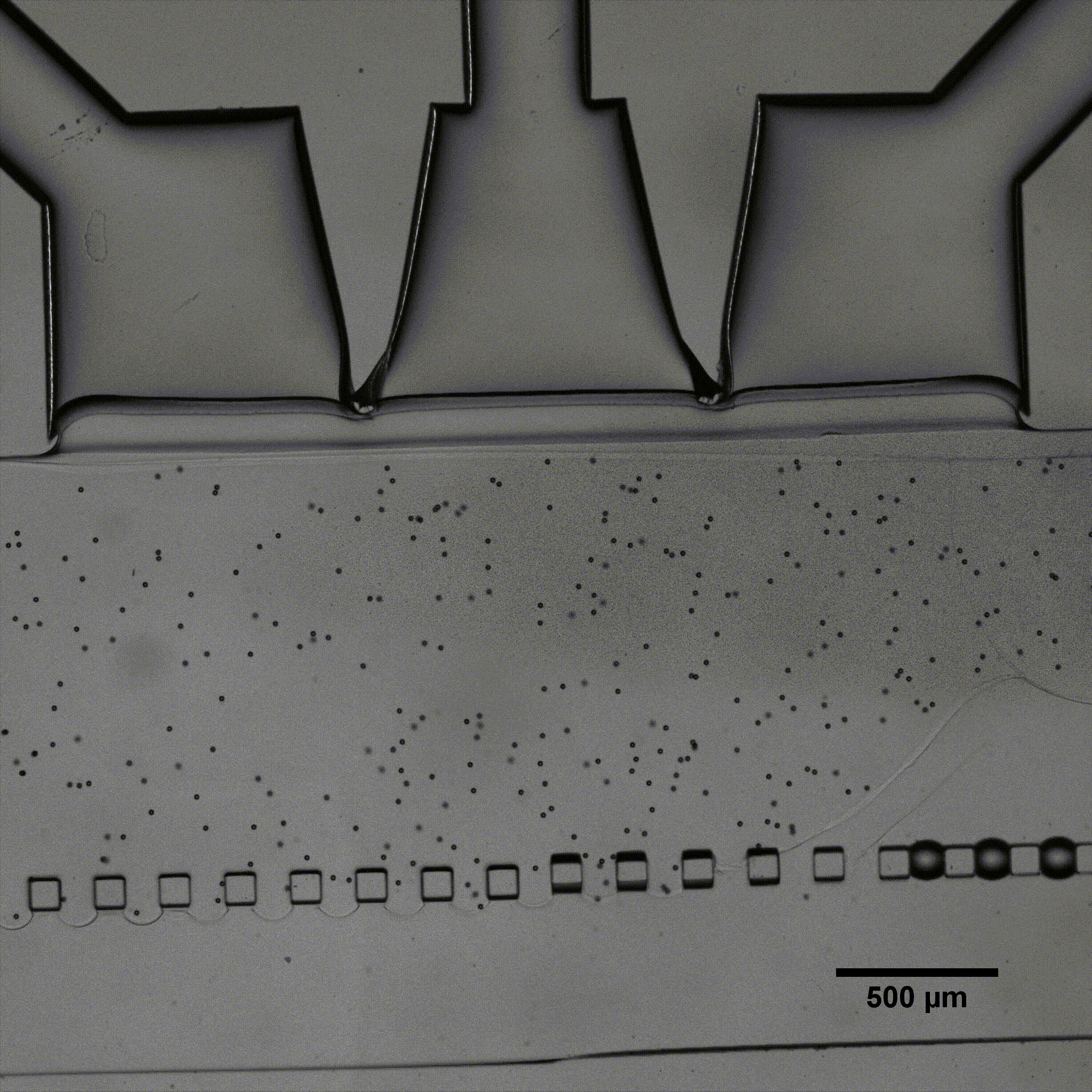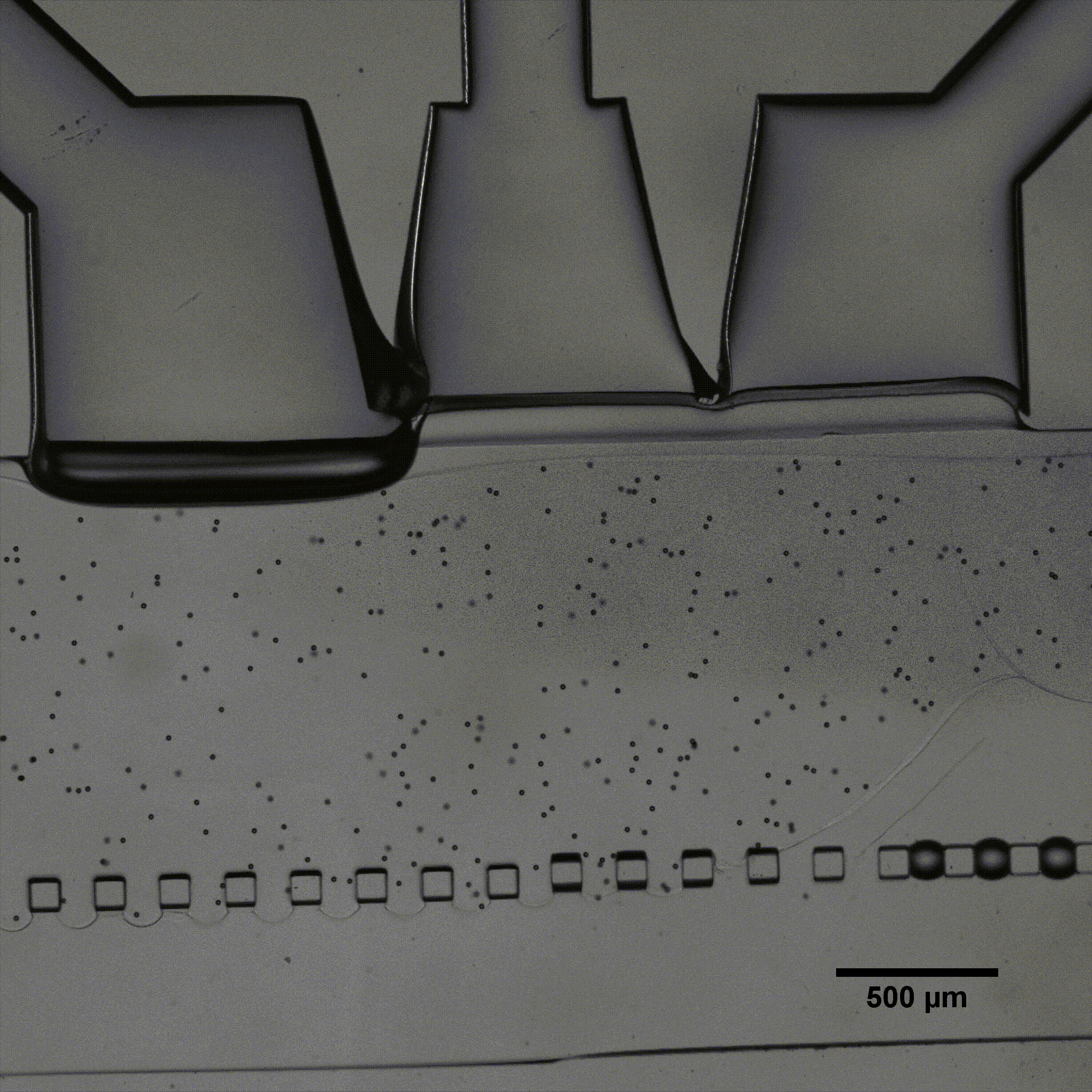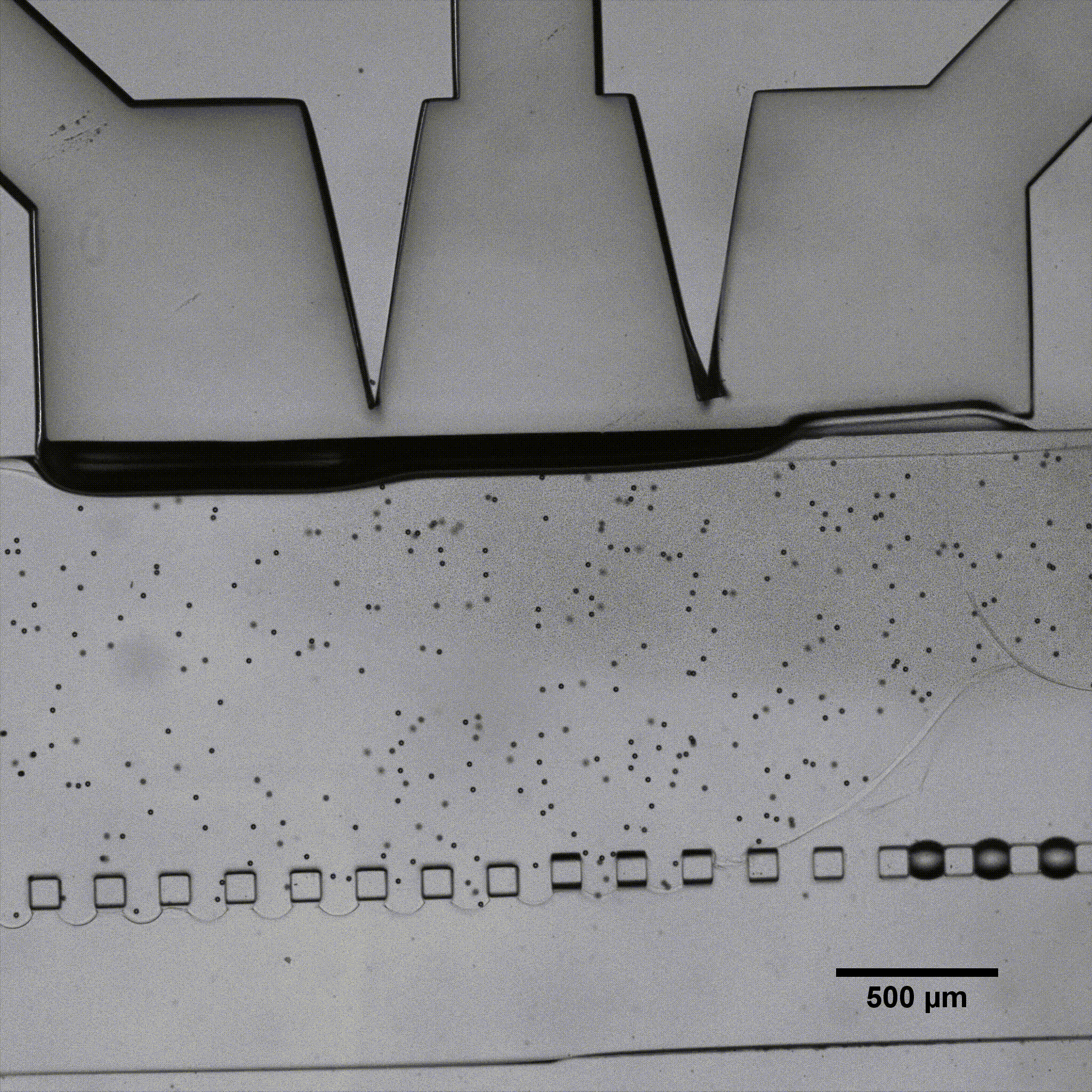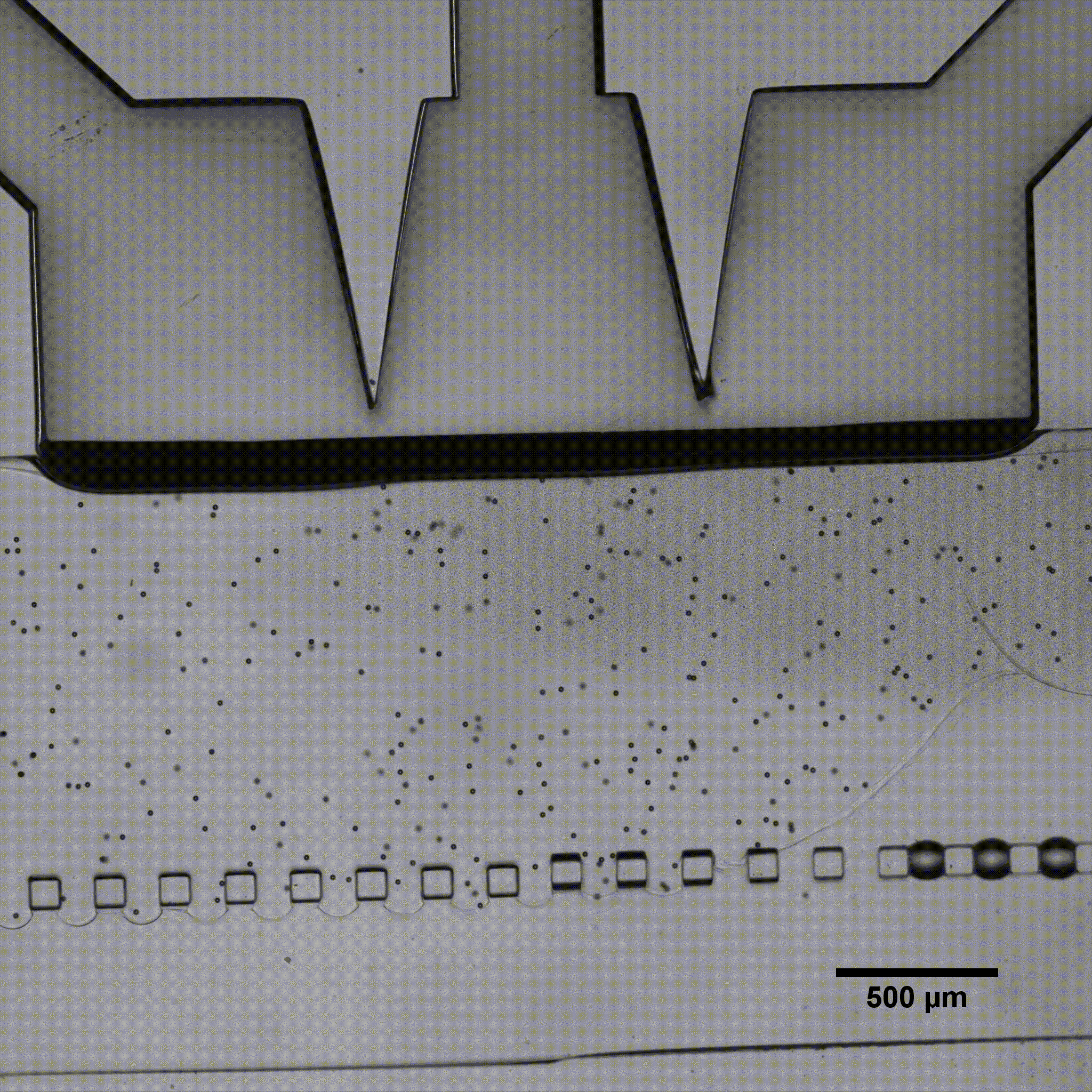
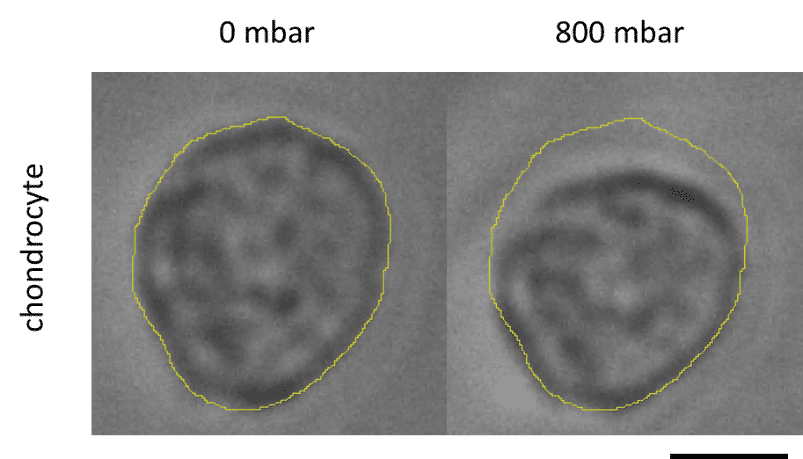
Elucidating how chondrocytes react to external stimuli (mechanical or chemical) is important to understand processes triggering cartilage diseases like osteoarthritis. To this end, various approaches, have been explored so far, while they all suffer from specific limitations.
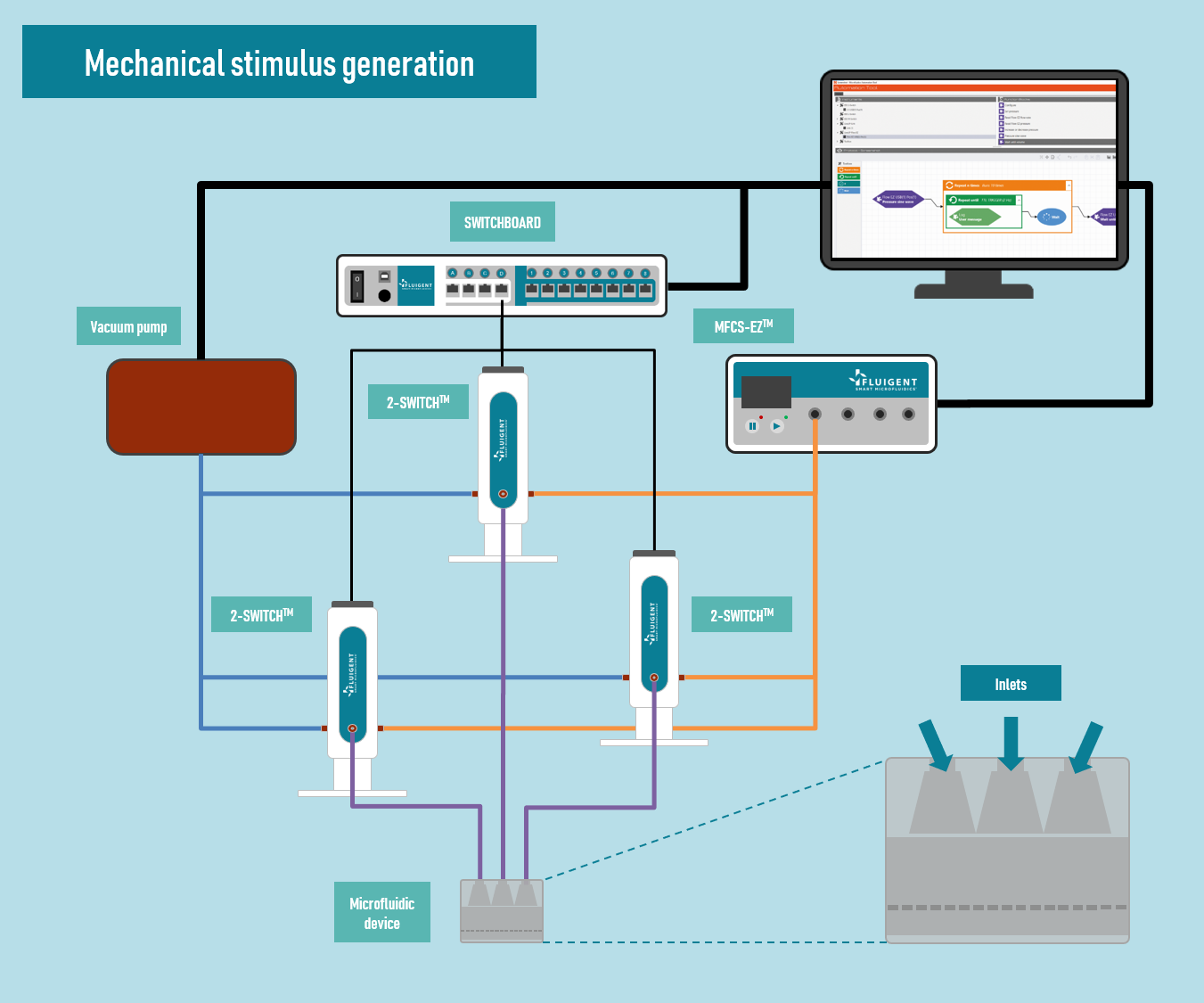
In this application note, we report on the use of Fluigent products to create complex mechanical stimulation patterns on 3D cell culture in a microfluidic platform, or so-called organ-on-a-chip device, with a specific focus on creating a cartilage-on-a-chip model.