Microbiome culture in droplet using dsurf surfactant
Biomillenia’s microbiome-on-a-chip technology uses droplet generation to create bacterial cell banks, evaluate microorganisms for desired phenotypes, and to find new routes to prevent and treat dysbiosis.
Most of the microfluidic droplet techniques require biocompatible surfactants to keep the droplets stable but this stability can be affected by the bacterial growth in microbiome culture in droplets. Hence, choosing the optimal surfactant for long-term incubation while being biocompatible for a wide spectrum of bacterial species is essential.
Here, the performance of three commonly used surfactants are compared at three different concentrations. The droplet stability over time and the droplet occupation rate is determined by encapsulating a microbial community derived from human skin.
Introduction
Why use a surfactant?
Surfactants are used for various reasons in many industries and applications. Firstly, they lower the surface tension of liquids, allowing better wetting, spreading, and penetration of substances. They can act as dispersants, helping to evenly distribute particles or droplets in solutions.
Additionally, surfactants can modify the interfacial properties between immiscible substances, such as oil and water, enabling processes like emulsification and enhancing oil recovery. Overall, surfactants play a crucial role in improving the efficiency and effectiveness of various industrial and consumer products such as microbiome in droplet experiments.
What is a microbial surfactant?
Microbial surfactants, also known as biosurfactants, are compounds produced by microorganisms that lower surface tension between substances. They possess hydrophilic and hydrophobic regions, enabling interaction with water and non-water substances. These surfactants have diverse compositions, including glycolipids, lipopeptides, phospholipids, and polymeric surfactants.
They offer advantages such as surface tension reduction, foaming ability, environmental compatibility, enhanced oil recovery, antimicrobial properties, and bioactivity. Their applications span agriculture, food processing, pharmaceuticals, cosmetics, bioremediation, and more. Microbial surfactants are an active area of research in biotechnology due to their unique properties and potential uses.
What are the advantages of biosurfactants over chemical surfactants?
Biosurfactants, or microbial surfactants, offer several advantages over chemical surfactants. They are environmentally friendly and derived from renewable resources, ensuring sustainability. Biosurfactants are non-toxic, posing minimal risks to humans and the environment.
They exhibit higher biodegradability, easily breaking down into simpler compounds. Their versatility allows customization for specific applications. Biosurfactants maintain functionality under extreme conditions, making them suitable for various industries. They synergize with microorganisms, enhancing their survival and activity.
Additionally, biosurfactants possess unique properties such as antimicrobial and anti-adhesive effects. While chemical surfactants still have their uses, biosurfactants provide a sustainable and eco-friendly alternative with diverse benefits, that makes it the perfect tool to perform microbiome culture in droplets.
How to Make Droplet-based Microbiome Culture
Reagents
Novec HFE7500 (3M) contains 5%, 2% or 0.5% (w/w) concentration of Competitor 1 surfactant, Competitor 2 Surfactant, or dSurf (Fluigent) .
Microbial sample
A microbial sample collected from the skin of healthy volunteers was used for microbiome culture in droplets generation. A general microbial skin sample, as opposed to a single strain like E. coli, was used here because a bacterial community consisting of many species better represents the experimental condition for microbiological research.
The microbial skin sample was prepared according to Biomillenia’s proprietary standard sample preparation workflow and was suspended in a standard medium for skin microbes containing 0.5% (v/v) TWEEN80. TWEEN80 is a lipophilic molecule in the aqueous phase that interferes with the droplet stabilizing characteristics of the surfactants.
Droplet generation
The droplets were prepared on Biomillenia’s proprietary microfluidic platform with PDMS chips. The two liquid phases were controlled during droplet generation by the use of high precision pressure pumps (Fluigent, Flow EZ).
Droplets were generated at frequencies of 7-10 kHz. The droplet volume was set to 20 pL. Droplets were collected and incubated in custom made vessels at 37 °C, allowing the droplets to be stored without exposing them to a direct gas interface.
Observation of the microbiome culture in droplets
From the droplet collection vessel, a small number of droplets were sampled to check for droplet stability and bacterial occupation at 0, 1, 3 and 7 days of incubation. For imaging by microscope, droplets were spread onto a monolayer surrounded by an oil-surfactant combination.
What material to perform droplet microfluidics for microbiome culture?
Partial results of droplet-based microbiome culture
The collected droplets were spread in Biomillenia’s proprietary observation chip for best imaging of microbial occupation in droplets after 7 days of incubation at 37 °C. As shown in the figure below, a monolayer of droplets surrounded by the respective oil-surfactant combination was imaged microscopically.
Surfactant concentrations of 2% and higher result in stable populations over 7 days at 37°C for dSURF and Competitor 2. While for Competitor 1,a concentration of 5% is required to reach similar results.
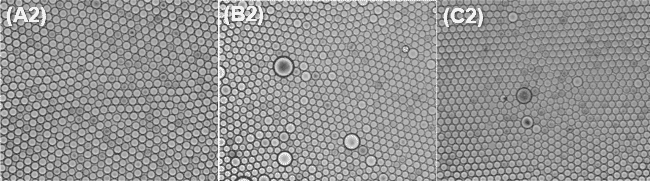
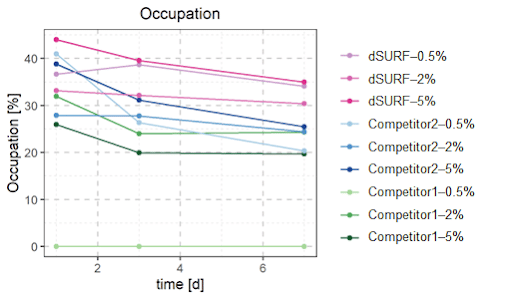
Bacterial occupancy was observed in the microbiome culture in droplets after 1, 3 and 7 days for each condition. A droplet is considered ‘occupied’ when it contains at least 3 bacteria. Since all the bacterial encapsulations were done in the same condition (same bacterial culture at the same concentration, same flow-rates, etc), one would expect the bacterial occupancy to be similar for each condition.
However, the choice of surfactant has a strong impact on the occupation rate, meaning a higher and more stable occupation rate is found with dSURF surfactants.
Conclusion
The stability of microfluidic emulsions strongly depend on the content of droplets and its interplay with the surfactant used. Hydrophobic compounds in the aqueous phase, along with microbial growth of various species, present far from ideal conditions for microfluidic droplets, but exemplify the experimental challenges encountered when microfluidic techniques are applied in microbiology. Hence, surfactants are needed to accommodate those complex sample characteristics.
In conclusion, dSURF surfactants for microbiome culture in droplets perform well compared to widely established microfluidic surfactants and are highly suitable for complex biological applications.

