Prostate Organoid Culture in Microbeads
Microbead-based microfluidics is a powerful technique that generates highly monodispersed picoliter-sized beads into a continuous phase. This method has been successfully adapted to cell culture to encapsulate cells in micron size hydrogel beads, constituting a process for the generation of organoid cultures in 3D matrices to mimic the complex in-vivo environment that supports cell physiological and pathological behaviors.
For instance, 3D epithelial organoids recapitulate numerous features of glandular tissues including the development of fully differentiated acini that maintain apicobasal polarity with the hollow lumen. Effective genetic engineering in prostate organoid culture would bring new insights in organogenesis and carcinogenesis.
The main advantages of this method are reduced costs related to miniaturization, high reproducibility, and high throughput screening capacities.
For more information on the application access the article:
Direct transfection of clonal organoids in Matrigel microbeads: a promising approach toward organoid-based genetic screens, Nucleic Acid Research, 1–13,
Introduction to Prostate Organoid culture
Advances in three-dimensional (3D) tissue organization models
The past decade has brought significant advances in prostate cancer (PCa) research. With the increased understanding of the origin and the molecular landscape of PCa, there has been an encouraging trend of precision medicine-based approaches to treat advanced PCa.
As we already know, tissues and organs are multicellular structures that self-organize in three dimensions (3D). Cells within a tissue, such as glandular tissue, interact with neighboring cells and the extracellular matrix (ECM) through biochemical and mechanical signals that maintain the specificity and homeostasis of biological tissues.
While traditional 2D cultures on rigid surfaces fail to reproduce cellular behavior in-vivo, 3D matrices are becoming increasingly popular supports for cell culture because they mimic the complex environment that supports the physiological functions of cells to better predict in-vivo responses, thus limiting the need for animal models.
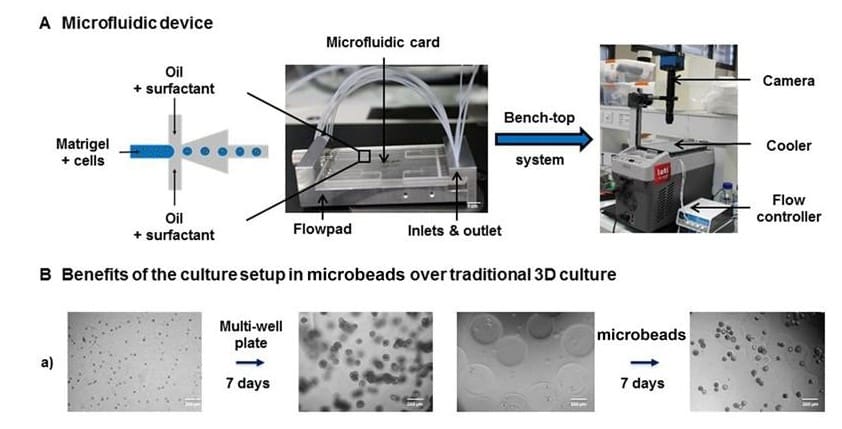
In collaboration with Leti, a technology research institute at CEA Tech.
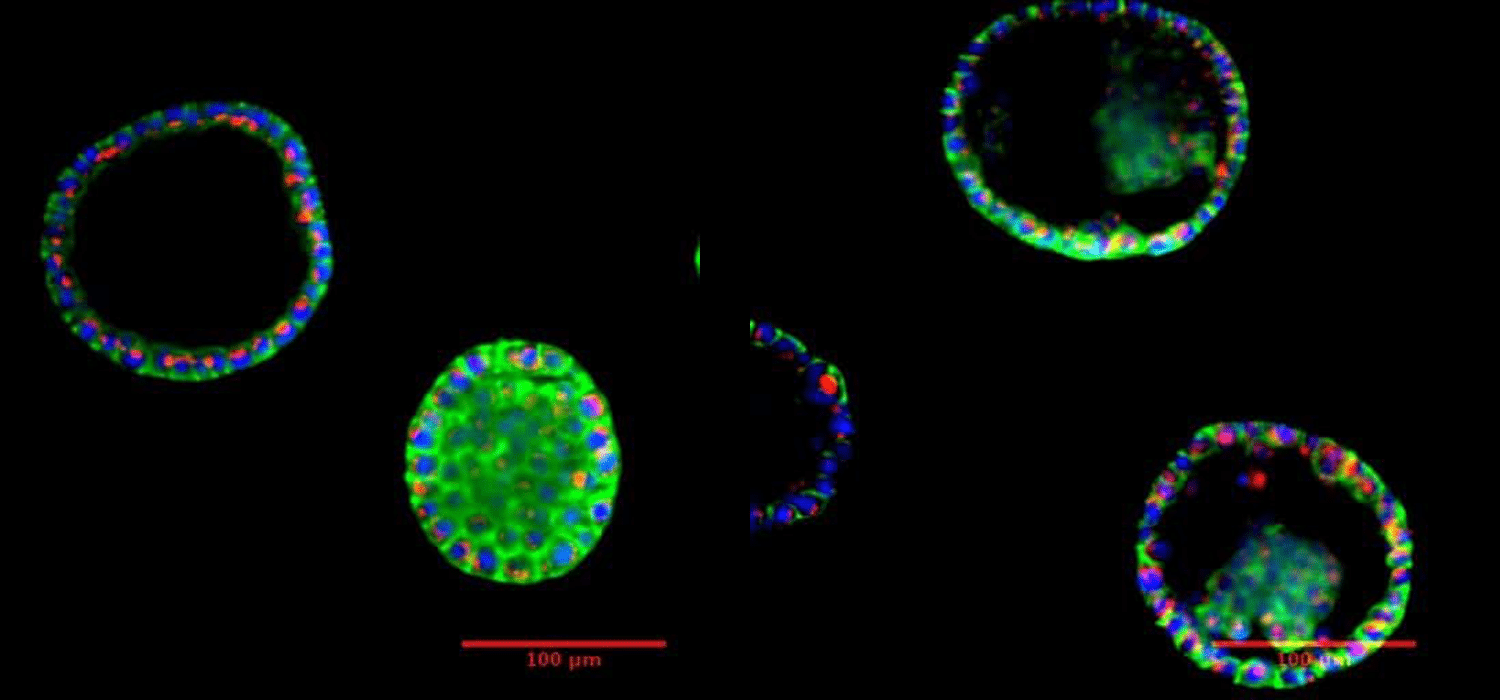
B. Prostate organoid after 7 days incubation. Visualization under fluorescent microscope.
Courtesy of Biomicrotechnology and Functional Genomics (BIOMICS), CEA, Grenoble, FRANCE.
In collaboration with Leti, a technology research institute at CEA Tech.
Organoids as Models for Prostate Cancer Research
Organoids are relevant models to mimic the complex in-vivo environment that supports cell physiological and pathological behaviors. For instance, 3D epithelial organoids recapitulate numerous features of glandular tissues including the development of fully differentiated acini that maintain apicobasal polarity with the hollow lumen. Therefore, researchers have been focusing on the production of prostate organoid culture to better understand the complexity of PCa initiation and progression.
Effective genetic engineering in prostate organoid culture would provide new insights into organogenesis and carcinogenesis, helping us to decipher the key genetic networks underlying epithelial differentiation and polarity, and allowing us to better understand how they may be altered in pathological states such as cancer.
Challenges in 3D Transfection of Organoids
However, direct 3D transfection on already-formed organoids remains challenging. One limitation is that organoids are embedded in the extracellular matrix and grow into compact structures that hinder transfection using traditional techniques. To address this issue, Laperrousaz, B. et al. (2018) have developed an innovative approach for transgene expression in 3D prostate organoid culture by combining single-cell encapsulation in Matrigel microbeads using a Fluigent microfluidic device and electroporation.
Laperrousaz, B. et al. (2018) demonstrated that direct electroporation of encapsulated prostate organoid cultures reach up to 80% of transfection efficiency when combining Fluigent’s technology for organoid generation and efficient 3D transfection. They were also able to validate the role of p63 and PTEN as key genes in acinar development in breast and prostate tissues confirming that this encapsulation and transfection method opens up new perspectives for flow-based high-throughput genetic screening and functional genomic applications.
Benefits of Organoid Culture
Single-cell embedded in microbeads: Each single microbead is considered as a single ‘bioreactor’ for 3D cell culture.
Clonal generation of organoids: Each single encapsulated cell gives rise to an organoid derived from clonal origin.
Monodispersity: The High Throughput (HT) formation of beads (2000 microbeads/min) of controlled size, shape, composition and cell distribution allows for the generation of homogeneous and ‘standardized’ organoids.
Reduced volume of matrix: 2 to 3 times less ECM (ExtraCellular Matrix) than traditional cultures. For example, 350 µl ECM (one well of a LabTek 4-chambers slides) produces 42.800 microbeads with a diameter of 250 µm). This is a great advantage of prostate organoid culture as one of the limitations of these tissues is the simulation of a matrix-embedded environment.
Easy handling: Recovery of organoids in culture media for further analysis.Storage: Microbeads with embedded organoids can be cryopreserved for long periods without altering the architecture and function of organoids.
Storage: Microbeads with embedded organoids can be cryopreserved for long periods without altering the architecture and function of organoids.
| 2D CELL CULTURE | STANDARD 3D CELL CULTURE | 3D CELL CULTURE IN MICROBEADS | |
|---|---|---|---|
| Biological relevance | Low | High | High |
| Control over 3D culture | / | Low | High |
| Easy handling | Yes | No | Yes |
| Clonality | No | No | Yes |
| Transfection efficiency | High | Low | High |
| Long term storage | Yes | No | Yes |
| High throughput | Yes | No | Yes |
| Cost | Low | High | Medium |
Organoid Culture Applications
Functional genomic studies: Controlled organoid generation combined to 3D iRNA-based electroporation in beads opens new perspectives for flow-based HT genetic screening and functional genomic application. As is the case with prostate organoid culture, the transfection efficiency is optimized by modulating microbead size and ECM concentration. The reduced amount of ECM surrounding organoids constitutes a permissive 3D environment that facilitates transfection. PubMed link ».
Tissue development and tumorigenesis: Collecting microbead-containing organoids at different stages allows users to perform a multi-omics analysis of organoid development or carcinogenesis. PubMed link »
Organoids / tumoroid-based drug assays: Flow-based strategies prove to be convenient for future HT Screenings in 3D models and identifying potential RNAi therapeutics. PubMed link »
3D Tool-box: Floating 3D organoids in beads can easily be aspirated, dispensed and sorted by large-particle fluorescence-assisted cell sorting. This flow-based technology opens up broad applications in the field of 3D culture. PubMed link ».


Expertises & Resources
Informative bibliography
[1] Laperrousaz, B., Porte, S., Gerbaud, S., Ville, H., Gidrol, X., Hourtane, V., & Picollet-D’hahan, N. (2018). Direct transfection of clonal organoids in Matrigel microbeads: a promising approach toward organoid-based genetic screens, Nucleic Acid Research, 1–13.
[2] Dolega, M. E., Abeille, F., Picollet-D’hahan, N., & Gidrol, X. (2015). Biomaterials Controlled 3D culture in Matrigel microbeads to analyze clonal acinar development. Biomaterials, 52, 347–357.
[3] Picollet-D’hahan, N., Dolega, M. E., Freida, D., Martin, D. K., & Gidrol, X. (2017). Deciphering Cell Intrinsic Properties: A Key Issue for Robust Organoid Production. Trends in Biotechnology, 35 (11), 1035–1048.
[4] Picollet-D’hahan, N., Dolega, M. E., Liguori, L., Marquette, C., Le Gac, S., Gidrol, X., & Martin, D. K. (2016). A 3D Toolbox to Enhance Physiological Relevance of Human Tissue Models. Trends in Biotechnology, 1–13.
Courtesy of Biomicrotechnology and functional genomics (BIOMICS), CEA, Grenoble, FRANCE.
In collaboration with Leti, a technology research institute at CEA Tech
The Organoids-on-Chip project has received funding from the EU’s H2020 research and innovation program (N°766884) (Read more)
